Genetic causes of cancer predisposition in children and adolescents
Introduction
Cancer is a multifactorial disease, with genetics being an important contributing etiologic factor. In particular, mutations influencing DNA repair genes, cell cycle regulators and cell-death pathways are the major genetic causes of malignancies (1).
The onset of cancer in an individual without an inherited cancer predisposition is due the acquisition of gene mutations in one or more somatic cells, altering the normal balance of cell proliferation, differentiation and death (2). On the other hand, an inherited cancer predisposition is a genetic condition that confers a higher likelihood of developing cancer, compared with the level of risk in the general population. In this case, the predisposing mutation(s) are passed from parents to offspring, and are therefore germline mutations (2). A particular condition that does not fall in these two categories is genetic mosaicism, where a mutation occurs in the early stages of embryonic development, leading to the presence of the mutation in only a subset of cells (3).
Inherited mutations can function in a dominant or recessive fashion, can confer different degrees of penetrance, and cause early- or late-onset disease, leading to marked variations in disease presentation within the cancer population (4). The majority of cancer predisposition genes act as tumor suppressors where mutations abrogate their function, while 10% of cancer predisposition genes (including genes encoding kinases such as ALK, KIT and MET) predispose to cancer through gain-of-function mutations (5).
The study of cancer genetics has profoundly helped in the understanding of cancer biology leading to better characterised malignancies, tailored targeted therapies, and the identification of individuals at high risk of cancer diagnosis (6). DNA-based genetic testing represents a crucial step in the identification of people with a high life-time risk of cancer, and for whom genetic counselling, screening and prevention may greatly improve either the chance of avoiding the onset of cancer, or the outcome of the disease (7). This review will provide an overview of conditions predisposing to cancer, with particular attention to the paediatric population, relevant genetic testing used to identify such conditions, and examples of how the presence of germline cancer-predisposing mutations can alter clinical care.
Cancer predisposition in young adults
Hereditary cancer accounts for up to 10% of all cancers, with hereditary breast-ovarian cancer syndrome and hereditary non-polyposis colon cancer being the best characterised hereditary cancers. Such tumors occur in families more often than would be expected by chance, often at an uncommonly early age, and indicate the presence of a gene mutation that increases the risk of cancer (8). Many mutated genes are known to play causal roles in different cancer syndromes, and these are listed in Table 1.
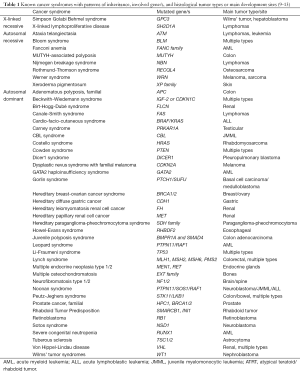
Full table
Hereditary breast-ovarian cancer syndrome is an autosomal dominant genetic condition caused by germline mutations in the BRCA1 and BRCA2 tumor suppressor genes (8,14). This disorder confers a higher risk of breast and ovarian cancer in women, and prostate cancer in men (14). In an analysis of over 8,000 index cases, the average cumulative risk in BRCA1-mutation carriers was 65% for developing breast cancer, and 39% for developing ovarian cancer by 70 years of age (15). The corresponding estimates for BRCA2 were 45% and 11%, respectively (15), although the penetrance of individual BRCA1 and BRCA2 gene mutations varies considerably (14). The relative risk of developing breast cancer is highest for BRCA1 mutation carriers between 30-39 years of age, whereas ovarian cancers tend to be diagnosed later (15). In contrast, relative risk estimates for breast cancer do not vary according to age for BRCA2 mutation carriers (15). For those individuals who are mutation carriers, several screening and primary prevention options have been suggested, including prophylactic surgery and chemoprevention (14). In the last few years, DNA sequencing has identified other rare loss-of-function variants that confer moderate risks of breast and/or ovarian cancer, in genes including PALB2 (16), ATM (17), BRIP1 (18), CHEK2 (19) and RAD51C (20), and the p53 inducible protein phosphatase PPM1D in a mosaic form (21).
Hereditary non-polyposis colon cancer, also known as Lynch syndrome, is another autosomal dominant cancer syndrome caused by genetic mutations in DNA mismatch repair genes (such as MLH1, MSH2, MSH6 and PMS2), that increase the risk of early development of a number of tumors including colorectal, stomach and pancreatic cancer (22). Defective mismatch repair leads to microsatellite instability and frame-shift mutations in genes involved in tumor initiation and progression (22). Lynch syndrome accounts for some 2-4% of colorectal cancers, and the reported lifetime risk of colorectal cancer varies between 38-100% for men with DNA mismatch repair mutations, and between 24-54% for women (23). Age-specific cumulative risks for colorectal cancer also vary considerably according to patient gender, and the specific mismatch repair gene that is mutated (23).
Cancer predisposition in children and adolescents
Compared with those tumor suppressor and DNA repair genes mutated in hereditary breast-ovarian cancer and hereditary non-polyposis colon cancer syndrome, mutations in other cancer predisposition genes can lead to cancer diagnoses at even younger ages. According to a recent publication by Rahman, a total of 114 cancer predisposition genes have been discovered in the past 30 years (24). Interestingly, 16 of these can cause both autosomal dominant and recessive conditions, with the recessive pattern of inheritance of a subset of these genes being associated with high risk of developing childhood cancer (24). Eight examples of cancer syndromes causing paediatric tumors will be described in the following sections. The first two (Fanconi anemia and Xeroderma pigmentosum) have a recessive pattern of inheritance, followed by four examples of dominant inheritance patterns (retinoblastoma, Li-Fraumeni syndrome, DICER1 syndrome and neurofibromatosis), one RAS mosaic condition, and Down syndrome.
Fanconi anemia
Fanconi anemia is characterised by congenital abnormalities, bone marrow failure, and a higher risk of developing myeloid and solid malignancies, such as squamous cell carcinoma and liver tumours, including adenoma and hepatocellular carcinoma (25,26). The median age for cancer diagnosis in Fanconi anemia patients has been reported as 16 years (25), and the cumulative probability of a solid tumor diagnosis is 75% by the age of 45 years (25). Indeed, there is a 500-fold increase in the incidence of head and neck squamous cell carcinoma in Fancomi anemia patients (27). The Fanconi anemia pathway counts 16 potentially mutated genes (including BRCA2, previously known as FANCD1) (28). Subsets of Fanconi anemia proteins are known to form complexes allowing protein ubiquitination, and subsequent DNA repair activation (28). Thus, while Fanconi anemia has been historically considered a disease of inter-strand cross-link sensitivity, Fanconi anemia proteins are now known to intersect with several distinct DNA repair pathways (28).
Xeroderma pigmentosum
A second example of an autosomal recessive disorder causing increased risk of skin cancers is Xeroderma pigmentosum, a rare condition characterized by increased sensitivity to UV light, and neurodegeneration. Xeroderma pigmentosum is the result of mutations in the XP nucleotide excision repair family of genes (29), and affected individuals have a more than 10,000-fold elevated risk of non-melanoma skin cancer, including melanoma (30).
Retinoblastoma
Retinoblastoma is a malignant tumor of the embryonic neural retina and the most common paediatric cancer of the eye. It usually affects young children, with more than 90% of cases diagnosed before 5 years of age (31). Mutations in the well described RB1 tumor suppressor are the genetic cause of retinoblastoma, and two main clinical forms have being described. Unilateral retinoblastoma accounts for approximately 75% cases and is due to sporadic RB1 mutations (31). The remaining 25% patients with bilateral disease carry a germline RB1 mutation (31), and are at high risk of developing second malignancies, especially sarcomas and melanoma. These individuals will require cancer surveillance throughout their life, and treatments will need to be tailored to avoid radiotherapy. In patients with bilateral retinoblastoma, only 25% inherit an RB1 mutation from an affected parent, whereas the remaining 75% of cases result from de novo RB1 mutations (31). In a small proportion of patients with germline RB1 mutations, retinoblastoma is also associated with pineoblastoma and other malignant midline supratentorial primitive neuroectodermal tumors (32). These cases have been described as trilateral retinoblastoma, and have historically shown very poor outcomes (32).
Li-Fraumeni syndrome
Li-Fraumeni syndrome is an autosomal dominant cancer syndrome caused by heterozygous germline mutations in the TP53 gene. It is characterised by the development of rare tumors in childhood and multiple common cancers of unexpectedly early onset in adulthood. In particular, 50% of patients with Li-Fraumeni syndrome develop at least one malignancy by 30 years of age, and one third of cancer survivors will develop multiple primary cancers over their lifetime (33).
TP53 is the most frequently mutated gene in human cancer and acquired TP53 mutations are also associated with diminished survival rates, increased resistance to chemotherapy and radiation, and elevated relapse rates (34). TP53 encodes a transcription factor with regulatory functions that promote DNA repair and tumor suppression. Most TP53 mutations are missense alterations clustered at codons 175, 248 and 273 in the central DNA-binding domain (35), and are considered to be highly penetrant. For example, TP53 mutations in the DNA-binding domain lead to a cancer diagnosis in 73% of men and nearly 100% of women during their lifetime (36), with cumulative 21% and 49% risks, respectively, of developing cancer before the age of 30 years (37). However, the R337H TP53 mutation only conferred an 11% risk of childhood adrenocortical cancer, as opposed to other classic Li-Fraumeni syndrome malignancies (38). Two other aspects of TP53 function may be relevant in children, namely telomere shortening and chromothripsis. Shorter telomeres were noted in TP53 mutation carriers that developed cancer either during childhood or adulthood, compared with non-affected controls (39). In addition, genome sequencing of medulloblastoma demonstrated associations between large catastrophic chromosome rearrangements, or chromothripsis, and mutations in TP53 (40).
DICER1 syndrome
Pleuropulmonary blastoma is a rare paediatric lung cancer of embryonal origin, and has been associated with germline DICER1 mutations (41). DICER1 encodes the endoribonuclease Dicer, a helicase enzyme which cleaves double-stranded RNA and pre-microRNA into small interfering RNA and microRNA, respectively, facilitating the activation of the RNA-induced silencing complex (42). DICER1 is therefore crucial for embryogenesis and early development (42,43). Forty different heterozygous germline DICER1 mutations have been reported in pleuropulmonary blastoma and other rare tumors of childhood, including cystic nephroma, ovarian Sertoli-Leydig cell tumor, embryonal rhabdomyosarcoma, supratentorial primitive neuroectodermal tumor and multinodular goiter (43).
Neurofibromatosis type I
Disorders described as neurofibromatoses represent three genetically distinct conditions which share an increased likelihood of cancer development, especially peripheral nerve sheath tumors and gliomas (44). Neurofibromatosis type I, the most common form of neurofibromatosis, is characterised by the development of benign neurofibromas on or around peripheral nerves (44), whereas in neurofibromatosis type II, the vestibulocochlear nerve develops schwannomas. In the third condition, Schwannomatosis, schwannomas develop on cranial, spinal and peripheral nerves (44).
Neurofibromatosis type I is associated with mutations in the NF1 gene that function in an autosomal dominant fashion (45). NF1 is responsible for the regulation of cell division through the inactivation of RAS-GTP, a fundamental step in the regulation of the RAS and PI3K pathways. In the absence of NF1, signaling is increased through these pathways, resulting in cell proliferation and inhibited apoptosis (45). Approximately half of all neurofibromatosis type I cases are diagnosed without a known family history, and are thought to represent sporadic NF1 mutations (46).
RASopathies and mosaic RAS mutations
A number of other developmental syndromes are associated with RAS pathway deregulation and are collectively referred to RASopathies (47). Somatic mutations in RAS subtypes occur frequently in human cancers, and germline mutations in these genes are the cause of complex syndromes with increased susceptibility to cancer, such as Noonan syndrome, Costello syndrome, Leopard syndrome and cardio-facio-cutaneous syndrome (48). The genetic characteristics of these conditions and their relationships with cancer development are listed in Table 1. While having some different features, RASopathies have common characteristics including dysmorphic appearance, skin abnormalities, congenital heart disease and an increased risk of malignancies (47). For example, embryonic rhabdomyosarcoma and neuroblastoma develop in up to 20% of Costello syndrome patients (49).
RAS mosaicism occurs when mutations are acquired during the post-zygotic stage, leading to the presence of the mutation in only a subset of cells (50). Often, this condition is identifiable because of the presence of birthmarks such as epidermal nevi or hemangiomas (51), and may confer a predisposition to cancer, according to Knudson’s “two hit” theory (50). Although malignant tumors have only rarely been reported in association with epidermal nevi, the risk of cancer might depend on the extent of mosaicism, the tissues involved, and the specific gene mutation. Also, children who are mosaic for RAS mutations but do not present with skin birthmarks may never be identified as such (50,52). Oncogene mutation mosaicism may therefore contribute to cancer causation to a greater extent than is presently appreciated.
Down syndrome
Down syndrome, or constitutional trisomy 21, is the most common human aneuploidy syndrome, and affected children have an elevated risk of acute leukemia which is up to 500-fold higher than that of the general population (53). Approximately 1% of children with Down syndrome develop spontaneously regressing transient myeloproliferative syndrome, acute myeloid leukaemia of Down syndrome, and/or B-lineage acute lymphoblastic leukaemia (53,54), which are commonly linked to dosage effects of chromosome 21 genes (54). Interestingly, acquired trisomy or tetrasomy 21 with the involvement of RUNX1 at chromosome 21q22 has also been described in many children with sporadic leukemias (55).
Trisomy 21 likely contributes to the development of GATA1 mutations, which are a feature of transient myeloproliferative syndrome, and also contribute to acute myeloid leukemia (AML) of Down syndrome (54). GATA1 mutations may also be responsible for the sensitivity of this disease to therapy, which is characterised by high cure rates (54). The molecular etiology of B-precursor acute lymphoblastic leukaemia in Down syndrome is very different, as GATA1 mutations are not detected, and clinical outcomes are worse than in patients without Down syndrome (54). B-precursor acute lymphoblastic leukaemia in Down syndrome is characterised by IKZF1 deletions and JAK2 tyrosine kinase mutations, the latter highlighting the possibility of targeted therapy for these patients (56).
Genetic testing and patient management
The guidelines for referring cancer patients to genetic testing have changed over the years. For example, since their description, the definitions of Li-Fraumeni and Li-fraumeni-like syndromes have been refined, notably by Chompret et al. (57), where TP53 mutations were expected to be identified in 20% of cases. In a subsequent study, 29% of patients meeting Chompret’s criteria (57) were identified as TP53 mutation carriers (58). DNA sequencing methods also have evolved from gene-specific tests using Sanger sequencing to whole exome or genome next-generation sequencing (59). With the use of next-generation sequencing, the number of recognised cancer predisposing mutations has dramatically increased, and has allowed genetic information to be associated with other medical conditions or drug responses (60).
Genetic testing of cancer patients and their relatives has helped to identify at-risk family members, thereby introducing surveillance and early intervention, and improving survival rates for familial cancers. As an example, a prospective study of asymptomatic germline TP53 mutation carriers showed 100% overall patient survival at 3 years in the enhanced surveillance group, compared with only 21% overall survival in the routine follow-up group (61). Given the complexity and gravity of Li-Fraumeni syndrome, guidelines and recommendations for patient management are extensive, and in addition to an annual physical examination, include age-specific breast cancer monitoring (or preventive mastectomy), colonoscopy every 2-5 years beginning no later than 25 years of age, and organ-targeted surveillance, based on cancers diagnosed in the family (62).
Point mutations in codon 918 of the RET oncogene have been identified in 98% of patients with multiple endocrine neoplasia type 2 (MEN2) (63). Thyroidectomy is recommended within the first 6 months of life, since codon 918 mutations are associated with early onset medullary thyroid cancers (63). For children with neurofibromatosis type I, annual surveillance for ocular signs of optic nerve glioma is recommended until 10 years of age, while annual blood pressure measurement is recommended for phaeochromocytoma detection (64). Individuals with neurofibromatosis type II are frequently screened to detect early acoustic neuroma, since surgery for hearing preservation has best chance of success when lesions are small at diagnosis (65). Similarly, the detection of RB1 mutations and early treatment of retinoblastoma has been shown to increase ocular retention and preserve vision (66), while screening for pineal tumors has increased the 5-year survival of trilateral retinoblastoma from 0% to 27% (67). Since the risk of developing Wilms’ tumor can be as high as 50% in children harbouring germline WT1 alterations, frequent renal ultrasounds are recommended from the age of syndrome diagnosis up to 5-7 years of age (68). Another example of successful intervention is the improved quality of life in children with familial adenomatous polyposis through enrolment on chemoprevention trials with celecoxib, which led to reduced colorectal polyp formation and delayed colectomies (69). Moreover, offering these patients prophylactic surgery significantly reduces mortality from colorectal cancer (70).
The growing use of genome-wide screening (71) and next-generation sequencing methods brings with it the possibility of identifying unexpected cancer-predisposing gene mutations, for example in members of unaffected families who have acquired de novo variations, as well as variations of unknown significance (72). These increasingly frequent scenarios highlight the importance of ethical approaches to the disclosure of incidental findings (73), and the psychological support needed by patients and families affected by cancer syndromes (74).
Conclusions
Although tumor predisposition underlies a comparatively small proportion of childhood cancer cases, the early age of diagnosis suggests that inherited cancer predisposition may underly a greater proportion of cases than is currently appreciated (69). The continued application of next-generation sequencing to childhood cancer patients and their family members will be expected to identify mutations in known cancer predisposition genes that have been previously associated with early onset adult cancer, as well as germline mutations in genes that are known to be somatically mutated in the same or other cancer types. Previously unrecognised tumor predisposition genes may also be uncovered through the study of patients with very rare cancers and/or unusual clinical presentation or features. Our current understanding of the genetic basis of tumor predisposition has already allowed the development of genetic tests that can assist in planning a life-long series of interventions that can include counselling, prevention, surveillance and early treatment, in the event of a cancer diagnosis. Expanding our knowledge of the genetic basis of cancer predisposition in children will have the exciting consequence of extending these benefits to larger numbers of patients and their families.
Acknowledgements
Funding: This publication was supported by funding to the Kids Cancer Alliance from the Cancer Institute NSW (Grant ID: 11/TRC/1-03), and from the University of Sydney.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vogelstein B, Kinzler KW. eds. The Genetic Basis of Human Cancer. New York: McGraw Hill, 2002.
- Anderson DE. The role of genetics in human cancer. CA Cancer J Clin 1974;24:130-6. [PubMed]
- Vijg J. Somatic mutations, genome mosaicism, cancer and aging. Curr Opin Genet Dev 2014;26:141-9. [PubMed]
- Frank SA. Genetic predisposition to cancer - insights from population genetics. Nat Rev Genet 2004;5:764-72. [PubMed]
- Dixit A, Yi L, Gowthaman R, et al. Sequence and structure signatures of cancer mutation hotspots in protein kinases. PLoS One 2009;4:e7485. [PubMed]
- Vucic EA, Thu KL, Robison K, et al. Translating cancer 'omics' to improved outcomes. Genome Res 2012;22:188-95. [PubMed]
- Narod SA. Screening for cancer in high risk families. Clin Biochem 1995;28:367-72. [PubMed]
- Banks KC, Moline JJ, Marvin ML, et al. 10 rare tumors that warrant a genetics referral. Fam Cancer 2013;12:1-18. [PubMed]
- Malkin D, Nichols KE, Zelley K, et al. Predisposition to pediatric and hematologic cancers: a moving target. Am Soc Clin Oncol Educ Book 2014.e44-55. [PubMed]
- Rao A, Rothman J, Nichols KE. Genetic testing and tumor surveillance for children with cancer predisposition syndromes. Curr Opin Pediatr 2008;20:1-7. [PubMed]
- Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 2014;123:809-21. [PubMed]
- Skokowa J, Steinemann D, Katsman-Kuipers JE, et al. Cooperativity of RUNX1 and CSF3R mutations in severe congenital neutropenia: a unique pathway in myeloid leukemogenesis. Blood 2014;123:2229-37. [PubMed]
- Smith MJ, Beetz C, Williams SG, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol 2014;32:4155-61. [PubMed]
- Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med 2010;12:245-59. [PubMed]
- Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003;72:1117-30. [PubMed]
- Rahman N, Seal S, Thompson D, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet 2007;39:165-7. [PubMed]
- Renwick A, Thompson D, Seal S, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 2006;38:873-5. [PubMed]
- Seal S, Thompson D, Renwick A, et al. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet 2006;38:1239-41. [PubMed]
- Meijers-Heijboer H, van den Ouweland A, Klijn J, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 2002;31:55-9. [PubMed]
- Meindl A, Hellebrand H, Wiek C, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet 2010;42:410-4. [PubMed]
- Ruark E, Snape K, Humburg P, et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature 2013;493:406-10. [PubMed]
- Drescher KM, Sharma P, Lynch HT. Current hypotheses on how microsatellite instability leads to enhanced survival of Lynch Syndrome patients. Clin Dev Immunol 2010;2010:170432.
- Barrow E, Hill J, Evans DG. Cancer risk in Lynch Syndrome. Fam Cancer 2013;12:229-40. [PubMed]
- Rahman N. Realizing the promise of cancer predisposition genes. Nature 2014;505:302-8. [PubMed]
- Alter BP. Cancer in Fanconi anemia, 1927-2001. Cancer 2003;97:425-40. [PubMed]
- Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood 2003;101:1249-56. [PubMed]
- Kutler DI, Auerbach AD, Satagopan J, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg 2003;129:106-12. [PubMed]
- Longerich S, Li J, Xiong Y, et al. Stress and DNA repair biology of the Fanconi anemia pathway. Blood 2014;124:2812-9. [PubMed]
- Lehmann AR, McGibbon D, Stefanini M. Xeroderma pigmentosum. Orphanet J Rare Dis 2011;6:70. [PubMed]
- Bradford PT, Goldstein AM, Tamura D, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet 2011;48:168-76. [PubMed]
- Rodriguez-Galindo C, Orbach DB, VanderVeen D. Retinoblastoma. Pediatr Clin North Am 2015;62:201-23. [PubMed]
- de Jong MC, Kors WA, de Graaf P, et al. Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol 2014;15:1157-67. [PubMed]
- Hisada M, Garber JE, Fung CY, et al. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998;90:606-11. [PubMed]
- Olivier M, Langerød A, Carrieri P, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res 2006;12:1157-67. [PubMed]
- Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat 2007;28:622-9. [PubMed]
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000;82:1932-7. [PubMed]
- Hwang SJ, Lozano G, Amos CI, et al. Germline p53 mutations in a cohort with childhood sarcoma: sex differences in cancer risk. Am J Hum Genet 2003;72:975-83. [PubMed]
- Figueiredo BC, Sandrini R, Zambetti GP, et al. Penetrance of adrenocortical tumours associated with the germline TP53 R337H mutation. J Med Genet 2006;43:91-6. [PubMed]
- Tabori U, Nanda S, Druker H, et al. Younger age of cancer initiation is associated with shorter telomere length in Li-Fraumeni syndrome. Cancer Res 2007;67:1415-8. [PubMed]
- Rausch T, Jones DT, Zapatka M, et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell 2012;148:59-71. [PubMed]
- Jaskiewicz L, Filipowicz W. Role of Dicer in posttranscriptional RNA silencing. Curr Top Microbiol Immunol 2008;320:77-97. [PubMed]
- Hill DA, Ivanovich J, Priest JR, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science 2009;325:965. [PubMed]
- Foulkes WD, Bahubeshi A, Hamel N, et al. Extending the phenotypes associated with DICER1 mutations. Hum Mutat 2011;32:1381-4. [PubMed]
- McClatchey AI. Neurofibromatosis. Annu Rev Pathol 2007;2:191-216. [PubMed]
- Gottfried ON, Viskochil DH, Couldwell WT. Neurofibromatosis Type 1 and tumorigenesis: molecular mechanisms and therapeutic implications. Neurosurg Focus 2010;28:E8. [PubMed]
- Ainsworth PJ, Rodenhiser DI, Costa MT. Identification and characterization of sporadic and inherited mutations in exon 31 of the neurofibromatosis (NF1) gene. Hum Genet 1993;91:151-6. [PubMed]
- Bezniakow N, Gos M, Obersztyn E. The RASopathies as an example of RAS/MAPK pathway disturbances- clinical presentation and molecular pathogenesis of selected syndromes. Dev Period Med 2014;18:285-96. [PubMed]
- Aoki Y, Matsubara Y. Ras/MAPK syndromes and childhood hemato-oncological diseases. Int J Hematol 2013;97:30-6. [PubMed]
- Siegel DH, Mann JA, Krol AL, et al. Dermatological phenotype in Costello syndrome: consequences of Ras dysregulation in development. Br J Dermatol 2012;166:601-7. [PubMed]
- Hafner C, Groesser L. Mosaic RASopathies. Cell Cycle 2013;12:43-50. [PubMed]
- Blatt J, Powell CM, Burkhart CN, et al. Genetics of hemangiomas, vascular malformations and primary lymphedema. J Pediatr Hematol Oncol 2014;36:587-93. [PubMed]
- Hafner C, Toll A, Real FX. HRAS mutation mosaicism causing urothelial cancer and epidermal nevus. N Engl J Med 2011;365:1940-2. [PubMed]
- Lange B. The management of neoplastic disorders of haematopoiesis in children with Down's syndrome. Br J Haematol 2000;110:512-24. [PubMed]
- Xavier AC, Taub JW. Acute leukemia in children with Down syndrome. Haematologica 2010;95:1043-5. [PubMed]
- Fonatsch C. The role of chromosome 21 in hematology and oncology. Genes Chromosomes Cancer 2010;49:497-508. [PubMed]
- Maloney KW, Taub JW, Ravindranath Y, et al. Down syndrome preleukemia and leukemia. Pediatr Clin North Am 2015;62:121-37. [PubMed]
- Chompret A, Abel A, Stoppa-Lyonnet D, et al. Sensitivity and predictive value of criteria for p53 germline mutation screening. J Med Genet 2001;38:43-7. [PubMed]
- Bougeard G, Sesboüé R, Baert-Desurmont S, et al. Molecular basis of the Li-Fraumeni syndrome: an update from the French LFS families. J Med Genet 2008;45:535-8. [PubMed]
- Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol 2008;26:1135-45. [PubMed]
- Rahman N. Mainstreaming genetic testing of cancer predisposition genes. Clin Med 2014;14:436-9. [PubMed]
- Villani A, Tabori U, Schiffman J, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: a prospective observational study. Lancet Oncol 2011;12:559-67. [PubMed]
- Schneider K, Zelley K, Nichols KE, et al. Li-Fraumeni Syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al. eds. GeneReviews®. University of Washington: Seattle, WA, 2013.
- Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and MEN type 2. J Clin Endocrinol Metab 2001;86:5658-71. [PubMed]
- Field M, Shanley S, Kirk J. Inherited cancer susceptibility syndromes in paediatric practice. J Paediatr Child Health 2007;43:219-29. [PubMed]
- Slattery WH 3rd, Fisher LM, Hitselberger W, et al. Hearing preservation surgery for neurofibromatosis Type 2-related vestibular schwannoma in pediatric patients. J Neurosurg 2007;106:255-60. [PubMed]
- Imhof SM, Moll AC, Schouten-van Meeteren AY. Stage of presentation and visual outcome of patients screened for familial retinoblastoma: nationwide registration in the Netherlands. Br J Ophthalmol 2006;90:875-8. [PubMed]
- Kivelä T. Trilateral retinoblastoma: a meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J Clin Oncol 1999;17:1829-37. [PubMed]
- Scott RH, Walker L, Olsen OE, et al. Surveillance for Wilms tumour in at-risk children: pragmatic recommendations for best practice. Arch Dis Child 2006;91:995-9. [PubMed]
- Schiffman JD. Hereditary cancer syndromes: if you look, you will find them. Pediatr Blood Cancer 2012;58:5-6. [PubMed]
- Tudyka VN, Clark SK. Surgical treatment in familial adenomatous polyposis. Ann Gastroenterol 2012;25:201-6. [PubMed]
- Schwarzbraun T, Obenauf AC, Langmann A, et al. Predictive diagnosis of the cancer prone Li-Fraumeni syndrome by accident: new challenges through whole genome array testing. J Med Genet 2009;46:341-4. [PubMed]
- Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet 2010;11:415-25. [PubMed]
- Netzer C, Klein C, Kohlhase J, et al. New challenges for informed consent through whole genome array testing. J Med Genet 2009;46:495-6. [PubMed]
- Lammens CR, Aaronson NK, Wagner A, et al. Genetic testing in Li-Fraumeni syndrome: uptake and psychosocial consequences. J Clin Oncol 2010;28:3008-14. [PubMed]


