Autosomal ring chromosomes in human genetic disorders
Introduction
Ring chromosomes as unusual circular chromosomes generally result from breaks at the ends of both chromosome arms with subsequent fusion of the broken ends to produce a continuous ring. Two types of ring chromosomes are present with varying clinical outcomes. The non-supernumerary ring replaces one of the normal homologs with a 46,(r) karyotype. A loss of genetic material is often reflected in some of these cases. Also observed are ends of broken chromosomes in which the telomere of one chromosome arm fuses with the telomere of its opposite chromosome arm. Fusion involving the subtelomeric sequences can also occur. Though rare, these “complete” rings in which no significant loss of genetic material is apparent, have been described in individuals with normal phenotypes (1,2).
The second type of ring, the supernumerary ring, is often very small and includes mainly pericentromeric material generally representing partial trisomies. These supernumerary rings with the karyotype 47,+(r) will not be included in this review.
Ring chromosomes have been represented in all human chromosomes. Only a few cases of parent to child transmission of ring chromosomes have been documented with 99% of rings arising sporadically (3). Nevertheless, the phenotype associated with ring chromosomes is expected to be variable depending on the chromosome involved and the extent of deletion present. An additional complication however is that of ring instability from sister chromatid exchanges during cell division. These lead to dicentric chromosomes and interlocking rings, breakage of which result in further chromosome aberrations and even loss of ring.
Ring chromosome behavior and the “ring syndrome”
A ring chromosome due its circular nature will be compromised and unstable at cell division. How a ring chromosome proceeds through mitosis will depend on whether sister chromatids in a ring chromosome break and join in a crossed manner. In the absence of any sister chromatid exchange at the breaks, separation of the ring chromatids at anaphase could occur without difficulty producing two similar equal sized rings with a centromere each and identical to the original (Figure 1).
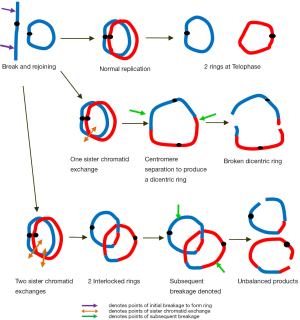
If a single crossover occurs between sister chromatids within a replicated ring, a large dicentric ring with two centromeres would result. At anaphase, the two centromeres move to opposite poles producing an anaphase bridge. Depending on where breakage and reunion occurs, unbalanced sister rings of unequal size with deleted or duplicated segments could form at telophase (Figure 1). Anaphase lagging may also occur leading to non-disjunction of the ring. In individuals carrying a ring chromosome, the ring has often been observed to be absent, duplicated, or dicentric in a small number of cells.
Should two sister strand exchanges occur, the replicated rings become intertwined forming interlocked rings (Figure 1). Breakage of these interlocked rings and their disruption at anaphase would lead to further aberrations including loss of the ring and through reunion of various broken ends, ring derivatives of various sizes. The genetic imbalance in these aneuploid cells with partial deletions, duplications, acentric fragments and ring loss would ensure their failure to survive in subsequent cell divisions. These cells are likely to be non-viable. Those that managed to survive in a mosaic form would likely be unbalanced enough to contribute unfavourably to the phenotype. Patients with ring chromosomes demonstrate mosaicism as a result of postzygotic ring instability and ring loss during cell division. The continuing production of aberrations in daughter cells referred to as “dynamic mosaicism” was first described by McDermott et al. (4).
Persistent generation of aneuploid cells and the subsequent loss of these cells would invariably predispose to significant growth deficiencies undermining the growth rate pre and post-natally. In each instance, these aneuploid aberrations are generated de novo and are not part of any established clone. This ring behaviour as such rather than any loss of genetic material prior to ring formation has been hypothesized to be critical to the growth failure (5). A recognizable pattern of anomalies characterized by growth retardation, no major malformations except for a few minor anomalies, mild cognitive impairment has been observed in patients with an autosomal ring. This phenotype associated with ring behaviour is independent of the chromosome involved and the term the “ring syndrome” has been proposed by Cote et al. (6). In an analysis of 207 case reports of patients with autosome rings, around 20% of the patients had extreme growth failure with no major malformations, features consistent with the “ring syndrome” (5). It was postulated that growth failure as the major physical abnormality of the ring syndrome is accounted for less by loss of genetic material at ring formation and more by aberrant ring behaviour subsequently generating loss or gain of genetic material (5). It was also suggested that ring size may determine instability with larger chromosome rings more unstable than smaller ones (5,7). The larger the chromosome involved, the more exposed it is to sister chromatid exchange and the more unstable. This leads to increasing in vivo cellular death with a higher probability of growth failure.
Recently, Rossi et al. (8) presented six patients with r(Jeny15) studied by aCGH and FISH and indicated that their data weakens the concept of the ‘‘ring syndrome’’. The IGF1R (insulin-like growth factor 1 receptor) gene at 15q26.3 is associated with growth and stature. Only probands found to have deletion of IGF1R in the r(Jeny15) had short stature in contrast to those with almost normal stature and non-deletion of the gene. It was felt that an imbalance of dosage sensitive genes involved in stature rather than ring instability would be responsible.
“Complete” rings and ring instability
Various reports of “complete” rings involving different chromosomes with intact telomeres or subtelomeric sequences at the fusion points have thrown support for ring instability as a contributing factor for the “ring syndrome” (1,9,10).
The three patients reported by Pezzolo et al. had rings involving chromosomes 4, 16 and 20 with telomere to telomere fusion occurring in ring formation and no visible deletion (9). They showed a phenotype consistent with the “ring syndrome” with failure to thrive, minor dysmporphic features and no major anomalies. With intact telomeres in these rings, the phenotype is attributed to the continuous generation and loss of aneuploid cells from structural instability of the ring in cell division rather than from small terminal deletions at ring formation. Two patients with complete ring chromosome (4) and ring chromosome (9) presented with severe symmetrical growth failure and normal psychomotor development with no major malformations (10). Intact telomeric sequences were present in the r(Jeny4) patient and absent in the r(Jeny9) patient. Severe anomalies and mental retardation were considered less likely in “complete” rings which have intact subtelomeric sequences with or without the distal telomeres (10).
Vermeech et al. described a 20-month-old boy with “complete” ring chromosome 7 with short stature, microcephaly and normal mental and motor development (1). Intact subtelomeric regions but no telomeric repeats were detected on FISH. Features of r(Jeny7) not apparent in this patient include mental retardation, facial asymmetry, limb and skeletal anomalies and skin lesions; haploinsufficiency of genes proximal to the subtelomeric regions were considered critical to the more severe phenotype seen in other r(Jeny7) patients.
Inheritance of ring chromosomes
Inheritance of a ring chromosome is uncommon. This is not unexpected as ring chromosomes are unstable during cell division with possible loss of the ring during meiosis. Ring chromosome carriers can be infertile reflecting a phenotype associated with alterations or loss of genetic material as a consequence of ring formation.
Meiotic transmission of rings has been shown in organisms like Drosophia and corn (11). Transmission of a ring chromosome is possible and can depend on any crossover between the ring and its homologue. The 46,(r) heterozygote at gametogenesis in normal disjunction without crossover and a 1:1 segregation results in gametes with either the ring or its normal homologue. Thus, half the conceptuses would carry the ring and the other half has normal karyotypes. However “dynamic mosaicism” in subsequent cell divisions could be lethal or lead to phenotype abnormalities. At meiosis, gamete instability invariably results from crossing over between the ring and its homologue with subsequent chromosome lagging from bridges at first or second meiosis. It is possible however, to recover a ring chromosome if two strand double crossovers occur (11). It has been noted that parent to child ring transmission are more often maternal in origin (12). Aberrant pairing failure of a ring 21 and its homologue leading to univalents at Meiotic metaphase I was observed in a ring bearing infertile male (13). Presumably the breakdown of spermatogenesis after this division would suggest that impaired spermatogenesis in male heterozygotes with a ring can produce reproductive failure.
The clinical manifestation of a familial ring chromosome in contrast to a sporadic ring is striking. Major malformation(s) and/or three or more minor anomalies were apparent in 47.4% of 194 patients with a sporadic ring (5). This is in contrast with a later study of 53 individuals with familial rings in which none had a major malformation (3). Only 6 of 48 patients had three or more minor insignificant anomalies. This 1991 review of inherited ring chromosomes from published cases involved 30 individuals (and two fetuses) and 23 carrier parents (21 mothers and two fathers). The phenotype of half of the offspring whether normal or abnormal, resembled closely that of the parent from whom the ring segregated (3). It is possible though that no significant deletion of chromosomal material was present in some of these cases. Combining parents and offspring, all 53 ring carriers were found to have few phenotypic abnormalities. More than a third was phenotypically and mentally normal with no major congenital malformations in any of the patients. Most of those with mental retardation were only mildly affected. A third of the nine patients with dysmorphism were children with trisomy 21. Of the 15 non-mosaic ring parents that produced non-mosaic offspring, four offspring showed a more severe clinical manifestation of the same ring. This finding is relevant when counseling clinically normal individuals who carry a ring chromosome.
There have been published reports of inheritance of ring chromosomes involving various chromosomes including 8, 13, 14, 17, 18, 20, 21 and 22 (3,14-16). A case of an inherited ring chromosome 8 syndrome was reported in a 6.5-year-old boy with short stature, microcephaly, mild mental retardation and behavioral problems (14). The mother had similar physical anomalies but intelligence was normal. FISH analyses on both mother and child showed intact subtelomeric sequences consistent with little deletion of euchromatic material. Their karyotypes involved loss of the ring in around 8% of cells.
Mother and daughter transmission of a ring 13 in which only subtelomeric 13q material was lost showed delayed language development in the daughter. The mother had short stature and borderline mental retardation (15). Transmission of an apparently “complete” ring 14 to two mentally subnormal female children and to a therapeutically aborted fetus was reported in a mother with low-normal intelligence (16). The elder child was microcephalic and at 30 months was diagnosed with degerative brain disease. The second child had prolonged convulsions and when assessed at 39 months was found to be at an 18 month development level. Interestingly, G-banding analysis showed the ring to be “open” as the satellite stalk associated with an active NOR (nucleolar organising region) was apparent and not giemsa staining. Satellite association of the stalk region of the ring with another acrocentric was also apparent. The mother was not retarded and could have escaped detection if not for the investigation in the children. Fertility was not affected though in males, ring chromosomes have been associated with sterility through spermatogenic impairment (16). The more severely affected offspring may have undetectable loss of material during exchange at meiosis between ring and its normal 14 homologue.
Ring 20 chromosomal mosaicism was reported in three carriers in two generations of a family (17). The ring 20 syndrome is characterized by mental retardation, epilepsy refractory to drug therapy and behavioral problems (18). Epilepsy is the most distinctive feature of the ring 20 syndrome, developing between 2 to 6 years of age and usually the first manifestation of the disorder. The male proband in the family developed normally but suffered seizures at 23 months with subsequent delayed development (17). The elder sister also appeared normal until at 2 years of age, myoclonic seizures were then observed. The mother was mentally normal with no signs of epilepsy. All three family members showed mosaicism for the ring 20 which had no visible genetic loss of material. Ring instability was apparent in the loss of the ring and the presence of dicentric and multiple rings. Higher frequencies of ring instability were seen in the siblings compared to the phenotypically normal mother. The question was raised as to how ring 20 mosaicism in the mother has been transmitted. It has been suggested that with the presence of a normal cell line, rather than the regular transmission of the ring, the chromosome 20 may be predisposed to terminal breaks or lesions which can be transmitted from parent to offspring. This would lead to possible de novo formation of a ring chromosome in the next generation.
Prenatal diagnosis of a female carrier of a familial r(Jeny21) who was of above average intelligence with a normal phenotype; revealed a twin pregnancy with a 46,XX,r(21)/45,XX,-21 karyotype in one fetus [77% r(Jeny21) cells] and a normal 46,XY karyotype in the second (19). Both fetuses were carried to term and were normal. Follow-up postnatal studies showed only a non-mosaic ring 21 karyotype in one twin. Ring instability can pose issues for genetic counseling. Both mother and brother of the female carrier also had the r(Jeny21) and were nondysmorphic. The r(Jeny21) was relatively stable with no aberrant forms of the rings evident in the carriers. Cultured amniocytes of the affected twin in which monosomy 21 was apparent showed three such cells across different cultures. It was concluded that the monosomy 21 cells were extra fetal in origin after it was established that this twin on postnatal follow up had a normal phenotype and was non-mosaic for the r(Jeny21) in blood lymphocytes. The case demonstrates the uncertainty and possible counseling issues that could arise in prenatal detection of mosaicism for ring chromosomes.
A familial r(Jeny21) inherited in mother and daughter has been characterized by aCGH to involve deletion of 3.4 Mb at the distal 21q22.3 end (20). The 32-year-old infertile daughter with a normal phenotype showed mosaicism with a 46,XX,r(21)/45,XX,-21/46,XX,dic r(Jeny21) karyotype. Ring instability generating variable levels of mosaicism was seen in loss of the r(Jeny21) and the presence of a dicentric r(Jeny21) in 4% and 2% of cells respectively. The family history was unremarkable except that the mother also phenotypically normal with the r(Jeny21) had two spontaneous abortions while the brother was developmentally delayed. Deletion of the long arm terminal region is consistent with breakage and reunion commonly associated with ring formation. Loss of the 3.4 Mb segment at 21q22.3 distal to the Down syndrome critical region in the phenotypically normal proband and mother would suggest the absence of dosage sensitive genes in the deleted segment. With reproductive performance an issue, molecular definition of the extent of the deletion in ring chromosomes and its inherent potential for instability, genetic counseling and prenatal diagnosis of ring chromosome carriers should always be considered (20).
An in vitro fertilized (IVF) pregnancy was referred for prenatal diagnosis because of an increased First Trimester Down syndrome screen risk of 1:21. The fetus was found to have a nonmosaic 46,XX,r(Jeny21)(p11.2q22.3) karyotype with deletion of 1.138 Mb at distal 21q on aCGH analysis. The mother with a 46,XX,r(Jeny21)(p11.2q22.3)/46,XX karyotype was a mosaic with 30% of r(Jeny21) cells. Counseling was difficult in defining a specific phenotype for the deleted segment as datasets describing similar imbalance were limited. A mild intellectual impairment was considered to be the worst likely scenario. However, a phenotypically normal female baby was delivered at term. This case emphasizes the importance of karyotyping in the management of couples referred for IVF (21).
Segregation of a r(Jeny22) was seen in three generations with the 24-year-old proband shown to have microcephaly, mild facial dysmorphism, an ataxia gait and mild mental retardation (22). Her father and three offspring had no abnormal features or mental retardation. All five individuals were mosaics with a normal cell line in 50-60% of the lymphocyte metaphases studied. The authors suggested that the more severe phenotype in the proband may be due to a predominance of defective cells in the brain.
Some ring chromosome cases of interest are presented below
Ring chromosome 1
The few patients presented have significant growth failure and mild mental retardation. A r(Jeny1) with little loss of chromosomal material and a size comparable to that of its normal homologue was observed in a 2-month-old girl who had facial dysmorphism and extreme growth retardation (23). Ring chromosome 1 also presented in a 9-year-old girl with severe dwarfism and mental retardation. She developed anemia and died of acute myeloid leukemia (24).
Ring chromosome 2
A 10-year follow up of a boy diagnosed at birth with r(Jeny2) and breaks at 2p25 and distal q37.3 revealed intrauterine growth retardation (IUGR), severe postnatal growth retardation, microcephaly and hypogenitalism (7). These findings were consistent with eight other cases of ring chromosome 2 reviewed that had similar breakpoints. Characteristic features of r(Jeny2) in these cases were short stature, microcephaly, mild mental retardation and few other anomalies, consistent with the concept of the “ring syndrome”.
Ring chromosome 3
An 18-year-old female investigated for short stature with a 46,XX,r (Jeny3)(p26.2q29) karyotype had mild facial dysmorphism and syndactyly of toes 2 and 3 (25). Her mild phenotype was explained by the “ring syndrome” with the 3p26.2 breakpoint distal to 3p25.3 recognised as critical to the deletion (Jeny3p) syndrome. The 3p-syndrome is characterized by growth and mental retardation, microcephaly, facial anomlies and polydactyly. These features were also seen in r(Jeny3) carriers with a similar deletion (26,27).
Ring chromosome 4
An 8-year-old boy with microcephaly, clinodactyly and growth retardation was shown to have a r(Jeny4) in 97% of cells analysed, the remaining cells had loss of the r(Jeny4) (28). Distal breakpoints with no visible deletion of short and long arms were noted. The instability of the large r(Jeny4) reflected in the high frequency of multiple ring configurations and the number of aneuploid cells was considered to be consistent with the “ring syndrome”.
Ring chromosome 6
A broad spectrum of phenotypic expression was seen in r(Jeny6)(p25q27) patients ranging from minimal physical anomalies and normal intelligence to severe mental and physical deficiencies. A prenatally diagnosed r(Jeny6) with ultrasound abnormalities was shown at amniocentesis to have 62% loss of the ring in a second cell line (29). It was continued to term and at birth, the female infant was microcephalic, had hypertelorism, a webbed neck, an imperforate anus and rocker bottom feet. It had feeding difficulties and failed to thrive and had a respiratory arrest at 4 months. The clinical variability of fourteen r(Jeny6) individuals when reviewed was shown to include growth failure, psychomotor retardation, eye and facial abnormalities and abnormal head size, features dependent on the extent of the deletion and the stability of the ring (30). A de novo r(Jeny6) presented in a female with mild dysmorphism and short stature but no developmental delay (31). There was little loss of euchromatic material as molecular investigations of the microdissected and amplified r(Jeny6) indicated breakpoints proximal to the telomeres with loss of around 200 kb from each arm of chromosome 6. Mitotic instability of the r(Jeny6) was stated to be responsible for the growth retardation and minor dsmorphism. She had a son with a normal karyotype.
Ring chromosome 10
A female newborn reported to have aganglionic megacolon and renal hypoplasia was found to have a r(Jeny10)(p15q26) (32). Physical examination at birth showed facial anomalies, short neck and severe hypotonia with reduced muscle mass. At 40 days, convulsions resistant to conventional therapy developed. The baby died in the third month with progressive renal failure. FISH analyses on lymphocytes and fibroblasts revealed monosomy 10 with ring loss in 96% of metaphases and 20% of interphase nuclei. Telomeres were also shown to be absent from the ring. It is suggested aganglionnosis of the colon could result from “dynamic somatic mosaicism” from aberrant r(Jeny10) behaviour and loss.
Ring chromosome 13
A group of r(Jeny13) patients with distal deletions involving bands 13q33 to 13q34 have a recognisable phenotype presenting with microcephaly, intellectual disability, growth retardation, facial dysmorphisms and genital anomalies (33). A 41-month-old boy with non-syndromic autism and a r(Jeny13) had a 2.11-Mb deletion at 13q34 on SNP microarray analysis. The deletion encompassing 23 genes including MCF2L and UPF3A, may provide possible candidate genes that could be associated with ASD (autistic spectrum disorders) (34).
Ring chromosome 14
The r(Jeny14) syndrome is characterized by a recognizable phenotype, consisting of distinct facial features, developmental delay, mental retardation, microcephaly, scoliosis and ocular anomalies that include abnormal retinal pigmentation, strabismus, glaucoma and abnormal macula (35). Drug resistant epilepsy is constant with early onset and generally shows intractable seizures (36). Contrary to the prevalence of generalized seizures previously reported, seizures may be partial, predominately focal, frontal, and temporal in origin; the degree of severity of the epileptic phenotype can negatively influence child cognitive development (36).
Zollino et al. compared 20 patients with r(Jeny14) and another nine patients with a linear 14q deletion (35). The first group had six cases with a complete ring and no apparent loss of chromosome material. A terminal 14q deletion, varying in size from 0.65 to 5 Mb, was detected in the remaining 14 cases. Of the second group of linear 14q deletions, three were proximal, varying in size from 4 to 7.2 Mb, and six distal, varying in size from 4.8 to 20 Mb. UPD (14) in which individuals inherit both copies of chromosome 14 from one parent instead of one copy from each parent, was absent in either group. By comparing patients with r(Jeny14) with those carrying a proximal or a distal deletion, it was found that seizures and microcephaly seem to be related to genes residing proximally on 14q11q13; a region which contains the FOXG1B gene known to be expressed in the developing fetal brain. Ring instability and haploinsufficiency of critical genes which could control and decrease the expression of genes contained in the adjacent more proximal 14q arm, have been proposed to explain the presence of seizures in r(Jeny14) syndrome (35).
Ring chromosome 15
Characteristic features of r(Jeny15) include growth retardation, variable mental retardation, microcephaly, hypertelorism and triangular facies (37). Severe growth deficiency is a common finding in r(Jeny15). It had been suggested that postnatal growth deficiency and severe IUGR could be related to the loss of the insulin-like growth factor I (IGF-1) receptor gene located at 15q26.3 affecting the number and structure of the growth receptors (38). A 4 year old boy with r(Jeny15)(p11.2q26.2) diagnosed at birth because of IUGR, had reduced IGF-1 levels. He had recombinant human growth hormone (rhGH) treatment and responded well with improved growth (39). Also reported was a 4.5-year-old girl with short stature, mental retardation, fifth finger clinodactyly, irregular café-au-lait spots, a r(Jeny15)(p11.2q26.3) karyotype without mosaicism and a normal IGF-1 gene. Low dose rhGH management showed a good response and should be considered in r(Jeny15) patients with short stature (40).
Ring chromosome 17
Ring chromosome 17 is rare with a variable phenotype depending on the presence or deletion of the Miller-Dieker critical region (MDCR). The more severe form consistent with the Miller-Dieker lissencephaly syndrome involves loss of MDCR on 17p13. The MDCR when present is associated with a mild phenotype which includes short stature, epilepsy, microcephaly, mental retardation and minor facial dysmorphisms (41).
Studies on two cases of mild r(Jeny17) syndrome led Surace et al. to hypothesize that telomere shortening can influence the phenotypic spectrum of this disease and contribute to the familial transmission of the mosaic ring (42). The patient in case 1 with facial dysmorphism, generalized café au lait skin spots, developmental delay and epilepsy had loss of the telomeres on the r(Jeny17). The proband with a milder phenotype in case 2 displayed increased shortening of the r(Jeny17) telomeric repeats. Her mother, also a r(Jeny17) mosaic with a normal cell line, presented only with café au lait skin spots and when compared to age matched controls, no significant reduction of telomere length in the r(Jeny17) was observed. The frequency of r(Jeny17) in peripheral blood of the patient and her mother was 72% and 44% of cells respectively. It was further suggested that as a consequence of the telomere shortening during embryogenesis, a circularization process is initiated leading ultimately to the formation of a ring chromosome. The stage in which this occurrence takes place is responsible for the different mosaicism rate.
Ring chromosome 18
Patients with r(Jeny18) have features resembling the 18p-syndrome, the 18q-syndrome or a combination of both (43). The phenotype of 18q-syndrome includes mental retardation, hypotonia, microcephaly, short stature, minor facial features and abnormal male genitalia while 18p-patients have speech delay, short stature, midline defects including holoprosencephaly, short neck, IgA deficiency (43). The study of five non-mosaic and two complex r(Jeny18) cases showed consistent loss of distal 18q and variable loss of 18p (43).
A mother with a 45,XX,-18/46,XX,r(Jeny18) karyotype had clinical features consistent with r(Jeny18), including short stature, cleft palate, and mild cognitive impairment (44). Monosomy 18 was seen in 11% of cells. Her six pregnancies with three karyotyped, all showed r(Jeny18) mosaicism with a monosomy 18 cell line present. The eldest daughter had clinical features resembling the mother except for absence of a cleft palate. Interestingly, the last pregnancy karotyped prenatally presented only with IUGR. The baby appeared normal phenotypically at birth but karyotype analysis was refused by the mother.
Ring chromosome 19
A 25-month-old girl showing areas of hypopigmentation along the lines of Blaschko was diagnosed with hypomelanosis of Ito (HI) [case 1 in (45)]. No other anomalies were observed and growth and development were normal. She presented with a ring chromosome 19 with intact subtelomeres. Mosaicism for r(Jeny19) was found in 20% of blood lymphocytes in the mother. HI has been suggested to be the result of chromosomal mosaicism. As ring chromosomes are unstable and associated with mosaicism the authors suggested that it could be possible that mosaicism in the patient’s neurocutaneous tissues may be related to her skin abnormalities. However, a skin sample was unavailable for study.
Ring chromosome 20
Ring chromosome 20 manifests as a rare refractory epilepsy syndrome that is characterized by a typical seizure phenotype consisting of complex partial seizures, a particular electroclinical pattern, cognitive impairment and absence of a consistent pattern of dysmorphology (46). In a study of 28 affected patients with r(Jeny20), two different forms of r(Jeny20) syndrome were recognised and involved mosaic or non-mosaic rings (18). Mosaic r(Jeny20) with a normal cell line and presumably postzygotic in origin, are formed by distal telomere to telomere fusion. Non-mosaic rings have distal deletions of one or both arms at the fusion point and are likely to be of meiotic origin. Mosaic r(Jeny20) patients have the less severe phenotype with later seizure onset and lesser likelihood of dysmorphism and intellectual disability. The mechanism underlying seizure disorders remains unclear, although it has been hypothesized that two epilepsy genes (CHRNA4 and KCNQ2), located on distal 20q, may be involved (18).
Ring chromosome 21
Depending on the loss of material from the long arm of chromosome 21, patients with r(Jeny21) have a variable phenotype ranging from normal to short stature, microcephaly, seizures, learning disabilities, heart defects, cleft lip and palate, and thrombocytopenia (20,47). A 2-year-old girl with persistent thrombocytopenia, syndactyly, and mild psychomotor and speech delay had a 46,XX,r(Jeny21)(p11q22.2)/45,XX,-21 karyotype with monosomy 21 and loss of the r(21)in 12% of metaphases (47). In contrast, FISH analysis on uncultured blood lymphocytes showed 86% monosomic 21 cells. Monosomy 21 was also found in 6% of cells on FISH analysis in a buccal smear study. aCGH analysis detected only monosomy 21 and if assessed in the patient without karyotyping, would have failed to identify the ring rearrangement. Parental karyotypes were normal. Ring formation involved breakage and reunion of the distal ends of short and long arms. The postzygotic instability of the r(Jeny21) would generate different degrees of mosaicism including loss of the ring chromosome. Varying levels of mosaicism in different tissues was suggested to further influence the phenotype of the patient (47).
Ring chromosome 22
Clinical features associated with ring chromosome 22 include developmental delay with severe speech disability, growth retardation, microcephaly, hypotonia, and facial dysmorphism (48). Depending on the extent of the terminal 22q deletion, the phenotype can overlap with the 22q13.3 deletion syndrome which consists of global developmental delay, absent or severely delayed speech, neonatal hypotonia, normal to accelerated growth, autistic behaviour and minor dysmorphic features. The SHANK3 (also known as PROSAP2) gene at 22q13.3 is critical to the phenotype. Support for this was the finding of a phenotypically normal r(Jeny22) individual with the ring chromosome not disrupting the SHANK3 gene (49). Undertaking a 22qter FISH analysis in patients with severe speech delay would be useful to identify 22qter cryptic deletions.
Atypical ring chromosomes
There have been reports in which ring chromosomes have been found to have additional aberrations and/or rearrangements other than deletions at the chromosomal ends (50,52-54).
A prenatal case determined at 22 weeks’ gestation because of hyperechogenic cardiac foci and intrauterine growth restriction revealed a mosaic r(Jeny21) male karyotype (50). The r(Jeny21) had a 5.03 Mb terminal deletion at 21q22.3 and was further identified on aCGH to include a 2.09 Mb interstitial deletion of 21q21.1-q21.2. The NCAM2 (Neural cell adhesion molecule 2) contained in the deleted segment was suggested to be a candidate gene for autism (50,51). The malformed fetus on termination was shown to have clinodactyly, short big toes, facial dysmorphism and micrognathia.
Knijnenburg et al. (52) reported an inverted duplication and terminal deletion in a patient with r(Jeny14). An additional duplication of 14q32.12->14q32.32 was identified next to the already detected deletion of 14q32.33->14qter, presumably as a result of an asymmetric breakage of a dicentric at meiosis II. In order to stabilise broken ends, it was suggested that ring chromosome formation itself can act as an alternative chromosome rescue mechanism next to telomere healing and capture, particularly for acrocentric chromosomes. Similarly, in 33 cases of ring chromosomes studied, seven were shown to not only have the expected terminal deletion but also a contiguous inverted duplication (53). It was also hypothesized that such inv dup del rearrangements may be stabilised by circularisation.
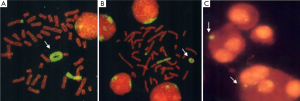
Yip et al. described an 11-year-old boy with short stature, learning difficulties, no facial dysmorphism and a ring chromosome 3 (54). This r(Jeny3)(p23q29) was formed by a break at 3p23 and its fusion with distal 3qter. A second rearrangement involved non-reciprocal translocation of the remaining 3p23->3pter segment to chromosome 6 at distal 6pter. There was no obvious loss of distal 6pter material as interstitial telomeres were present on the der(Jeny6). The one chromosome 3 was involved in two rearrangements; presumably ring formation as a rescue mechanism occurred to help stabilise broken ends of the 3p23-3qter segment (52,54). The patient’s relatively mild phenotype and lack of any obvious facial dysmorphism except for growth and mental retardation is indicative of the “ring syndrome”. Variable sized ring chromosome 3 and micronuclei representing displaced chromatin derived from r(Jeny3) of this patient are shown in Figure 2.
Conclusions
The many ring cases reviewed provide examples of the variable nature, size and imbalance of autosomal ring chromosomes and their severity in patients. It would be essential for new cases of a ring chromosome when found, to be provided with a more precise molecular definition to determine the exact size and location of any chromosomal imbalance. Consideration should also be given to the secondary imbalance due to ring instability when counseling patients as any strict genotype-phenotype correlation may not be possible. For ring chromosome carriers, in case of pregnancy, prenatal diagnosis should always be considered.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Vermeesch JR, Baten E, Fryns JP, et al. Ring syndrome caused by ring chromosome 7 without loss of subtelomeric sequences. Clin Genet 2002;62:415-7. [PubMed]
- Guilherme RS, Meloni VF, Kim CA, et al. Mechanisms of ring chromosome formation, ring instability and clinical consequences. BMC Med Genet 2011;12:171. [PubMed]
- Kosztolányi G, Méhes K, Hook EB. Inherited ring chromosomes: an analysis of published cases. Hum Genet 1991;87:320-4. [PubMed]
- McDermott A, Voyce MA, Romain D. Ring chromosome 4. J Med Genet 1977;14:228-32. [PubMed]
- Kosztolányi G. Does "ring syndrome" exist? An analysis of 207 case reports on patients with a ring autosome. Hum Genet 1987;75:174-9. [PubMed]
- Cote GB, Katsantoni A, Deligeorgis D. The cytogenetic and clinical implications of a ring chromosome 2. Ann Genet 1981;24:231-5. [PubMed]
- Lacassie Y, Arriaza MI, Vargas A, et al. Ring 2 chromosome: ten-year follow-up report. Am J Med Genet 1999;85:117-22. [PubMed]
- Rossi E, Messa J, Zuffardi O. Ring syndrome: still true? J Med Genet 2008;45:766-8. [PubMed]
- Pezzolo A, Gimelli G, Cohen A, et al. Presence of telomeric and subtelomeric sequences at the fusion points of ring chromosomes indicates that the ring syndrome is caused by ring instability. Hum Genet 1993;92:23-7. [PubMed]
- Sigurdardottir S, Goodman BK, Rutberg J, et al. Clinical, cytogenetic, and fluorescence in situ hybridization findings in two cases of "complete ring" syndrome. Am J Med Genet 1999;87:384-90. [PubMed]
- Palmer CG, Hodes ME, Reed T, et al. Four new cases of ring 21 and 22 including familial transmission of ring 21. J Med Genet 1977;14:54-60. [PubMed]
- MacDermot KD, Jack E, Cooke A, et al. Investigation of three patients with the "ring syndrome", including familial transmission of ring 5, and estimation of reproductive risks. Hum Genet 1990;85:516-20. [PubMed]
- Tulloch WS, Newsam JE. Studies on human meiotic chromosomes from testicular tissue. Lancet 1966;1:679-82. [PubMed]
- Le Caignec C, Boceno M, Jacquemont S, et al. Inherited ring chromosome 8 without loss of subtelomeric sequences. Ann Genet 2004;47:289-96. [PubMed]
- Bedoyan JK, Flore LA, Alkatib A, et al. Transmission of ring chromosome 13 from a mother to daughter with both having a 46,XX, r(13)(p13q34) karyotype. Am J Med Genet A 2004;129A:316-20. [PubMed]
- Riley SB, Buckton KE, Ratcliffe SG, et al. Inheritance of a ring 14 chromosome. J Med Genet 1981;18:209-13. [PubMed]
- Back E, Voiculescu I, Brünger M, et al. Familial ring (20) chromosomal mosaicism. Hum Genet 1989;83:148-54. [PubMed]
- Conlin LK, Kramer W, Hutchinson AL, et al. Molecular analysis of ring chromosome 20 syndrome reveals two distinct groups of patients. J Med Genet 2011;48:1-9. [PubMed]
- Melnyk AR, Ahmed I, Taylor JC. Prenatal diagnosis of familial ring 21 chromosome. Prenat Diagn 1995;15:269-73. [PubMed]
- Bertini V, Valetto A, Uccelli A, et al. Ring chromosome 21 and reproductive pattern: a familial case and review of the literature. Fertil Steril 2008;90:2004.e1-5.
- Mazzaschi RL, Love DR, Hayes I, et al. Inheritance of a Ring Chromosome 21 in a Couple Undergoing In Vitro Fertilization (IVF): A Case Report. Case Rep Genet 2011;2011:158086.
- Stoll C, Roth MP. Segregation of a 22 ring chromosome in three generations. Hum Genet 1983;63:294-6. [PubMed]
- Kjessler B, Gustavson KH, Wigertz A. Apparently non-deleted ring-1 chromosome and extreme growth failure in a mentally retarded girl. Clin Genet 1978;14:8-15. [PubMed]
- Bobrow M, Emerson PM, Spriggs AI, et al. Ring-1 chromosome, microcephalic dwarfism, and acute myeloid leukemia. Am J Dis Child 1973;126:257-60. [PubMed]
- McKinley M, Colley A, Sinclair P, et al. De novo ring chromosome 3: a new case with a mild phenotype. J Med Genet 1991;28:536-8. [PubMed]
- Narahara K, Kikkawa K, Murakami M, et al. Loss of the 3p25.3 band is critical in the manifestation of del(3p) syndrome: karyotype-phenotype correlation in cases with deficiency of the distal portion of the short arm of chromosome 3. Am J Med Genet 1990;35:269-73. [PubMed]
- Wilson GN, Pooley J, Parker J. The phenotype of ring chromosome 3. J Med Genet 1982;19:471-3. [PubMed]
- Freyberger G, Wamsler C, Schmid M. Ring chromosome 4 in a child with mild dysmorphic signs. Clin Genet 1991;39:151-5. [PubMed]
- Dawson AJ, Marles SL, Harman CR, et al. Prenatal diagnosis of ring chromosome 6. Prenat Diagn 1995;15:872-4. [PubMed]
- Peeden JN, Scarbrough P, Taysi K, et al. Ring chromosome 6: variability in phenotypic expression. Am J Med Genet 1983;16:563-73. [PubMed]
- Höckner M, Utermann B, Erdel M, et al. Molecular characterization of a de novo ring chromosome 6 in a growth retarded but otherwise healthy woman. Am J Med Genet A 2008;146A:925-9. [PubMed]
- Calabrese G, Franchi PG, Stuppia L, et al. A newborn with ring chromosome 10, aganglionic megacolon, and renal hypoplasia. J Med Genet 1994;31:804-6. [PubMed]
- Xu F, DiAdamo AJ, Grommisch B, et al. Interstitial Duplication and Distal Deletion in a Ring Chromosome 13 with Pulmonary Atresia and Ventricular Septal Defect: A Case Report and Review of Literature. N A J Med Sci 2013;6:208-12.
- Charalsawadi C, Maisrikhaw W, Praphanphoj V, et al. A case with a ring chromosome 13 in a cohort of 203 children with non-syndromic autism and review of the cytogenetic literature. Cytogenet Genome Res 2014;144:1-8. [PubMed]
- Zollino M, Seminara L, Orteschi D, et al. The ring 14 syndrome: clinical and molecular definition. Am J Med Genet A 2009;149A:1116-24. [PubMed]
- Giovannini S, Marangio L, Fusco C, et al. Epilepsy in ring 14 syndrome: a clinical and EEG study of 22 patients. Epilepsia 2013;54:2204-13. [PubMed]
- Butler MG, Fogo AB, Fuchs DA, et al. Two patients with ring chromosome 15 syndrome. Am J Med Genet 1988;29:149-54. [PubMed]
- Roback EW, Barakat AJ, Dev VG, et al. An infant with deletion of the distal long arm of chromosome 15 (q26.1----qter) and loss of insulin-like growth factor 1 receptor gene. Am J Med Genet 1991;38:74-9. [PubMed]
- Nuutinen M, Kouvalainen K, Knip M. Good growth response to growth hormone treatment in the ring chromosome 15 syndrome. J Med Genet 1995;32:486-7. [PubMed]
- Xu F, Zou CC, Liang L, et al. Ring Chromosome 15 syndrome: Case Report and Literature Review. HK J Paediatr 2011;16:175-9. (New Series).
- Surace C, Piazzolla S, Sirleto P, et al. Mild ring 17 syndrome shares common phenotypic features irrespective of the chromosomal breakpoints location. Clin Genet 2009;76:256-62. [PubMed]
- Surace C, Berardinelli F, Masotti A, et al. Telomere shortening and telomere position effect in mild ring 17 syndrome. Epigenetics Chromatin 2014;7:1. [PubMed]
- Stankiewicz P, Brozek I, Hélias-Rodzewicz Z, et al. Clinical and molecular-cytogenetic studies in seven patients with ring chromosome 18. Am J Med Genet 2001;101:226-39. [PubMed]
- Yardin C, Esclaire F, Terro F, et al. First familial case of ring chromosome 18 and monosomy 18 mosaicism. Am J Med Genet 2001;104:257-9. [PubMed]
- Hermsen MA, Tijssen M, Acero IH, et al. High resolution microarray CGH and MLPA analysis for improved genotype/phenotype evaluation of two childhood genetic disorder cases: ring chromosome 19 and partial duplication 2q. Eur J Med Genet 2005;48:310-8. p. [PubMed]
- Giardino D, Vignoli A, Ballarati L, et al. Genetic investigations on 8 patients affected by ring 20 chromosome syndrome. BMC Med Genet 2010;11:146. [PubMed]
- Militti L, Alfonsi M, Palka C, et al. Mosaic Ring Chromosome 21 in a Patient with Mild Intellectual Disability not evidenced by Array-Cgh. J Genet Syndr Gene Ther 2013;4:207.
- De Mas P, Chassaing N, Chaix Y, et al. Molecular characterisation of a ring chromosome 22 in a patient with severe language delay: a contribution to the refinement of the subtelomeric 22q deletion syndrome. J Med Genet 2002;39:e17. [PubMed]
- Jeffries AR, Curran S, Elmslie F, et al. Molecular and phenotypic characterization of ring chromosome 22. Am J Med Genet A 2005;137:139-47. [PubMed]
- Chen CP, Lin YH, Chou SY, et al. Mosaic ring chromosome 21, monosomy 21, and isodicentric ring chromosome 21: Prenatal diagnosis, molecular cytogenetic characterization, and association with 2-Mb deletion of 21q21.1eq21.2 and 5-Mb deletion of 21q22.3. Taiwan J Obstet Gynecol 2012;51:71-6. [PubMed]
- Molloy CA, Keddache M, Martin LJ. Evidence for linkage on 21q and 7q in a subset of autism characterized by developmental regression. Mol Psychiatry 2005;10:741-6. [PubMed]
- Knijnenburg J, van Haeringen A, Hansson KB, et al. Ring chromosome formation as a novel escape mechanism in patients with inverted duplication and terminal deletion. Eur J Hum Genet 2007;15:548-55. [PubMed]
- Rossi E, Riegel M, Messa J, et al. Duplications in addition to terminal deletions are present in a proportion of ring chromosomes: clues to the mechanisms of formation. J Med Genet 2008;45:147-54. [PubMed]
- Yip MY, MacKenzie H, Kovacic A, et al. Chromosome 3p23 break with ring formation and translocation of displaced 3p23-->pter segment to 6pter. J Med Genet 1996;33:789-92. [PubMed]


