Evaluation of cerebral function in high risk term infants by using a scoring system based on aEEG
Introduction
Amplitude integrated electroencephalogram (aEEG) is derived from the original EEG signal by processing to digitally amplify, smooth, rectify, and compress allows for the evaluation of brain function in real time and in over long periods (1), which is considered a method that cannot interfere with routine medical care and the output can be interpreted by nonexperts. In the past four decades, aEEG has been refined and is now widely used in the neonate intensive care unit. It has been used for predicting the neurodevelopment outcome in infants with encephalopathy after asphyxia; monitoring seizures and effects of anticonvulsants; and as a marker for following the neurological maturation of the brain of premature infants (2-9).
Now the technique also has been explored in monitoring series of critical ill infants by different pathogenesis. Such as severe hypoglycemia can cause aEEG background depressing and seizures showed in an experimental setting (10), but mild hypoglycemia does not change the signal of aEEG significantly (11). And such as moderate unconjugated hyperbilirubinemia cause a transient but delayed suppression of aEEG in preterm infants (12). Furthermore, aEEG could also be used predicted the outcomes of the patients with encephalopathy due to bacterial meningitis (13) and display an upward shift of the lower aEEG amplitude margin or high-frequency burst-suppression (BS) pattern in patients with inborn errors of metabolism (14).
Although there is growing support for the use of aEEG in NICU, the utility for the common neonatal encephalopathy other than HIE has not been well-studied. The comprehensive interpretation of aEEG is limited by challenges in reliably quantifying and summarizing data of encephalopathy caused by different pathogenesis, and the relationship between outcomes and early aEEG monitoring has yet to be determined. Accordingly, our study aims to investigate the relationship between the early aEEG changes of the full term newborn at risk of neurological compromise, and to propose a clinically applicable an aEEG scoring system that will be able to predict an adverse neurodevelopmental outcome early and quantitatively.
Materials and methods
Patients and entry criteria
A total of 81 term newborns at high neurological risk that were treated in a level-III NICU of the Children’s Hospital of Zhejiang University School of Medicine between February 2008 and April 2012 were enrolled in the present study. The study was approved by the local ethics committee and the parents of all patients enrolled gave informed consent.
The criteria for the diagnosis hypoxia and ischemia encephalopathy (HIE) (15) includes signs of intrauterine asphyxia, as indicated by late decelerations on fetal monitoring or by meconium staining of the amniotic fluid; evidence of metabolic acidosis (pH less than 7.00, base deficit greater than or equal to 12 mmol/L); early onset encephalopathy; and multi system organ dysfunction. HIE was graded according to Sarnat and Sarnat (16). The criteria of hypoglycemic brain damage was according by Burns et al. (17) and Mao et al. (18) including: (I) one documented episode of hypoglycemia (blood or plasma glucose concentration of 2.6 mmol/L) associated with acute neurologic dysfunction during the first postnatal week; (II) symptoms included poor feeding, hypothermia, jitteriness, hypotonia, irritability, lethargy, seizures, cyanosis, and apnea; (III) obvious brain injury changes under magnetic resonance imaging (MRI). Acute bacterial meningitis was diagnosed according to the criteria described in avery’s diseases of newborn (19), the gold standard for diagnosis of meningitis is the analysis of the cerebrospinal fluid. Acute bilirubin encephalopathy was diagnosed according to Johnson et al. (20): with unconjugated hyperbilirubinemia, irritability, and muscular hypertonia accompanied with opisthotonos, and at least one of the following three symptoms, namely lethargy, decreased feeding, hypotonia or hypertonia, high-pitched cry, or abnormal brainstem auditory evoked potential. Inborn error metabolism disease was diagnosed according to corresponding symptoms, along with hyperlactacidemia or hyperammonemia, and determined by tandem mass spectrometry and gas chromatography-mass spectrometry, genetic testing was performed for further confirmation.
Congenital infections, major malformations, multiple dysmorphic features and/or chromosomal abnormalities were excluded.
aEEG and analysis
The aEEG recording (NicoletoneTM Monitor) was applied by the attending neonatologist. Skin was cleaned with a dermabrasive cream and covered with collodium before disc electrodes were placed and fixed in their positions at FP1, FP2, C3, C4, and reference Fz, using the international ten-twenty system of electrode placement. The upper limit of tolerated impedance was 5 kΩ. Frequencies lower than 2 Hz or higher than 20 Hz were filtered. The aEEG signal was displayed on a semi-logarithmic scale at a speed of 6 cm/h (21). The aEEG tracings were reviewed by two clinicians who were blinded to the clinical data. The following channel pairs were used in the present study: FP1-FP2, FP1-C3, FP2-C4, and C3-C4 for aEEG, C3-C4 for burst density and inter-burst interval (IBI). Monitoring was installed as soon as possible and continued until patients were stable or died.
Classification of aEEG background pattern
According to the criteria described by Hellström-Westas et al. (22): (I) continuous normal voltage (CNV): continuous activity with lower (minimum) amplitude around >5 µV and maximum amplitude >10 µV; (II) discontinuous voltage (DC): discontinuous background with minimum amplitude variable, but mainly below 5 µV, and maximum amplitude over 10 µV; (III) BS: discontinuous background with minimum amplitude without variability at 0-1 [2] µV, and bursts with amplitude >25 µV. BS+ means burst density ≥100 bursts/h, and BS– means burst density <100 bursts/h; (IV) continuous low voltage (CLV): continuous background pattern of very low voltage (around or below 5 µV); and (V) inactive, flat (FT): mainly inactive (isoelectric tracing) background below 3-5 µV.
Classification of sleep-wake cycling (SWC) (22)
SWC in the aEEG is characterized by smooth sinusoidal variations, mainly in the minimum amplitude. The broader bandwidth represents discontinuous background activity during quiet sleep (trace alternant EEG in term infants), and the narrower bandwidth corresponds to more continuous activity during wakefulness and active sleep. (I) No SWC: no cyclic variation of the aEEG background; (II) imminent/immature SWC: some, but not fully developed, cyclic variation of the lower amplitude, but not developed as compared to normative gestational age representative data; (III) developed SWC: clearly identifiable sinusoidal variations between discontinuous and more continuous background activity with cycle duration 20 min.
Seizures (6)
Epileptic seizure activity in the aEEG is usually seen as an abrupt rise in the minimum amplitude, usually accompanied by a simultaneous rise in the maximum amplitude and often followed by a short period of decreased amplitude. The classification of epileptic seizures was as follows: (I) single seizure; (II) repetitive seizures (≥3 seizure patterns during a 30-minute period); and (III) status epilepticus (SE) continuous seizure pattern for ≥30 minutes, presenting as a “saw tooth pattern” or as continuous increases of the lower and upper margins.
Scoring system based on aEEG for term infants at risk for neurological sequelae
The aEEG tracing provided data on the aEEG background pattern, epileptic electrical activity and SWC and was scored as shown in Table 1. The three variables were combined create a cerebral function monitoring scores which was calculated by summarizing the scores of the three column. The cerebral function monitoring scores were classified three grades as ≤4, >4-≤8, and >8.
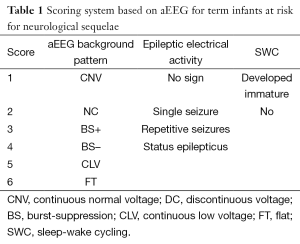
Full table
Follow-up study
Follow-up study was performed by a physiotherapist or neurologist who was blinded to clinical course. Neurodevelopmental outcomes were assessed by using Bayley Scales of Infant Development, Second Edition (23) when the infants were 12 to 18 months of age. Cerebral palsy was defined as a psychomotor development index (PDI) <70 and, mental deficiency was defined as a mental development index (MDI) <70 and confirmed by Bayley intelligence text. Outcomes were classified into four groups: without deficit (normal); cerebral palsy and/or mental deficiency or with motor or mental retardation (abnormal); death prior to discharge; infants were lost to follow up.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software package. Spearman’s rank correlation analysis and Chi-square test were performed to investigate the correlation between aEEG tracing and neurodevelopment outcome, P<0.05 was considered as statistically significant. Sensitivity, specificity, positive and negative predictive values were calculated in order to evaluate the aEEG’ predictivity for neurological evolution. The area under the receiver operator characteristics (ROC) curve was calculated to evaluate the value of scoring system based on aEEG in predicting neurological outcome in term infants. Youden’s index was calculated to determine the cut-off value and area under curve (AUC) >0.9 was considered to confer high diagnostic value.
Results
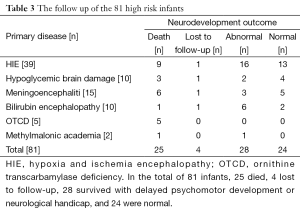
A total of 81 critical ill infants completed aEEG tracings, the mean gestational age was 38.2 (36-42+3) wks, and mean birth weight was 3,202 g (1,800-4,500 g). Monitoring started at 10 [2-20] hours of life, or after admitted in NICU. The mean correct age was 39.5 (36-42+5) when started to monitoring and the mean duration of monitoring was 47.8 [15-96] hours. Twenty-five of the infants died and 48 infants required ventilation during the study period. A total of 39 infants were diagnosed with moderate or severe HIE. Ten infants were diagnosed with hypoglycemic brain damage. A total of 15 infants were diagnosed with acute bacterial meningoencephalitis. Ten infants were diagnosed with acute bilirubin encephalopathy. Five infants were confirmed to have ornithine transcarbamylase deficiency (OTCD), and two infants with methylmalonic academia.
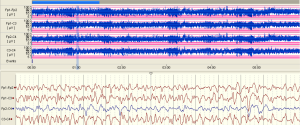
The examples of the representative tracing analysis were shown in Figures 1-4. The aEEG features of the total infants (including background pattern, SWC and epileptic electrical activity) and neurodevelopmental outcomes for the 81 infants at 12- to 18-month are displayed in Tables 2 and 3.
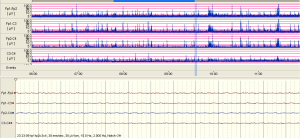
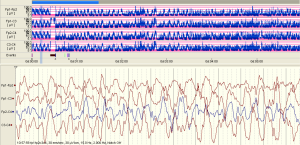
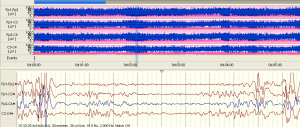

Full table
Full table
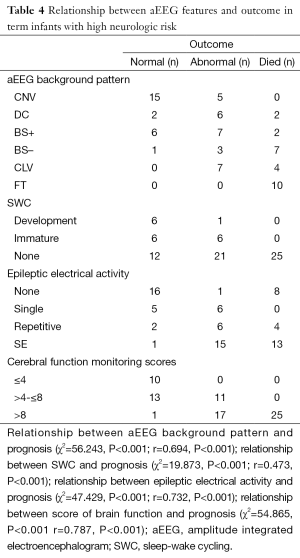
The relationship between the follow-up study and the aEEG tracing changes (Table 4) revealed that the infants’ aEEG background pattern were FT, CLV, or BS– who often had poor outcome (died or abnormal): 10 infants with FT lasting for more than 24 hours all died; 4 of the 11 with CLV and 7 of the 11 with BS– also died, and the remaining 7 infants with CLV, 3 infants with BS– had delayed psychomotor development, just 1 infant with BS– was healthy. The aEEG background pattern was CNV in 20 infants, all these infants survived: 15 were healthy, 5 had delayed psychomotor development (Table 4).
Full table
In another hand, the aEEG features of the infants that died in neonatal period showed as follows: ten infants with FT, two infants with CLV/SE (status epileptics in addition to CLV), two infants with CLV/repetitive seizures, six infants with BS–/SE, one infant with BS–/repetitive seizures, one infant with BS+/SE, one infant with BS+/repetitive seizures, two infants with DC/SE. which revealed that pathological aEEG background pattern including in addition to SE or repetitive seizures is a predictor for poor outcome (Table 4).
The thirdly the result also showed that 6 of 7 infants who had developed SWC were recovered and had good outcome while 58 infants were found without SWC, only 12 of these infants had good outcome (Table 4).
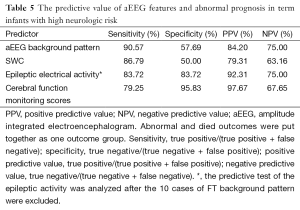
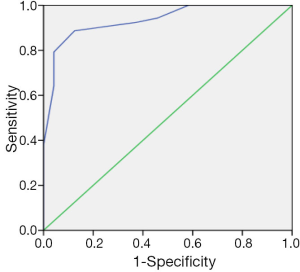
There was a positive correlation between aEEG background pattern, SWC, epileptic electrical activity and neurodevelopment outcome; which was confirmed by spearman correlation test. Similar correlation was also found between the cerebral function monitoring total scores and neurological outcome, and the scoring system has a higher r value than other separated entities (Table 4). Predictive test showed the cerebral function monitoring total scores also has a higher specificity, PPV, but lower sensitivity compared with the separate entities such as background pattern, seizures and SWC (Table 5). The area under the ROC curve was calculated to evaluate the value of scoring system based on aEEG in predicting neurological outcome, and the AUC was 0.934 (P<0.001) (95% confidence interval, 0.878-0.990). Youden’s index was calculated, and the value of 7.5 was chosen as the cut-off value that could provide a high sensitivity and specificity simultaneously (Figure 5).
Full table
Discussion
In the present study, we analyzed the data of aEEG in the acute stage of 81 infants with high neurological risks which was not limited to brain damage after asphyxia. The other diagnoses included hypoglycemia brain injury, acute bacterial meningoencephalitis, acute bilirubin encephalopathy, and inborn errors of metabolism. Our results showed that the abnormal aEEG changings of severe neurological disorders of term infants are similar to that of infant’ with severe HIE.
The characteristics of EEG and aEEG vary greatly for infants with different gestational age. Burdjalov and colleagues (24) developed a scoring system which consists of Co (continuity of the recording), Cy (presence of SWC), LB (lower border amplitude score), and B (bandwidth), to assess objectively the developmental maturation of the neurologically unpaired premature. However, for full-term infants or infants with same gestational ages of those brain-injured neonates, there is still no a valuable tool to quantify changes of aEEG, the evaluation cerebral function by aEEG is mainly based on single parameter in previous researches. Such as Toet Mc and Hellström-Westas group showed the aEEG background pattern early within 6-h after birth could predict the severity of HIE (3), Osredkar et al. showed that sleep wake cycling was a predictor of good outcome when appearing within 36 hours, and associated with higher scores on Griffith Developmental Quotient (25).
Three parameters including aEEG background, electro physiologic maturity and electrographic seizures should be all evaluated in the interpretation of aEEG described by El-Dib et al. (26). Amplitude suppression (voltage suppression) was considered to be the result of brain damage (27), discontinuous EEG signal with a broader aEEG bandwidth and the BS pattern (a flat lower margin interrupted by brief spikes of higher amplitude activity) were also suggesting of encephalopathy (22). Shany et al. speculated the background pattern is more sensitive than voltage measurements when evaluating the cerebral function (28). We chose the background pattern as the first component of the scoring system in the study. Furthermore, electrophysiological maturity is intimately connected to the development of SWC. The emergence of cycling depends on the level of integration of cerebral function (25). So the cycling is another important parameter for evaluating the cerebral function. The thirdly is presence or classification of electrographic seizures, seizures would likely to increase neuronal injury, and have an adverse effect on the neurodevelopmental outcome, especially recurrent seizures and SE (29-31). Accordingly we developed the new scoring system incorporates the above three individual component variables of the aEEG features to evaluate objectively and comprehensively the cerebral function of full-term infants who were at increased risk of abnormal neurological events or adverse neurodevelopment outcomes.
The results from correlation study revealed the cerebral function monitoring scores we proposed in this paper has a higher r value then the three individual items. The score system is feasible for clinical using by neonatologist; the lower score stands for better neurodevelopmental outcome and the higher score for bad ones. And we also found that the infant with the score higher than 8 (the cut-off value the ROC curve was 7.5) was with poor outcome. So this scoring system may be more useful than single parameter such as background pattern or electrical activity or SWC because it can afford comprehensive and quantifying data.
The limitation of the score system may decrease the sensitivity (79.25%) of prediction because the compound sum score added from serial inter-relative items. So cerebral function monitoring scores system has a higher specificity, PPV but lower sensitivity compared with the separate entities such as background, seizures and SWC. It can be thought of that separate entities of aEEG and the total CFM score have their respective advantages and disadvantages, and also have certain guiding clinically.
Conclusions
Our findings suggested that using aEEG could provide useful information of cerebral function of infants with high nervous system risks in NICU, and the new scoring system we proposed for term infants may be as a semi-quantitative tool evaluating neonates following high risk events regardless of their cause, it could help guide the extent of the clinical work and refine long-term follow up of these patients.
Acknowledgements
Thanks to the following people for their assistant with data collection (Dr. Yu Bao and the practitional nurses in NICU of children’s Hospital of Zhejiang University School of Medicine particularly Xiaoying Chen and Haihong Zhu) and for their technical support of our study (Dr. Anita Kharbteng and Dr. Weijian Li). We are also appreciative of Prof. Lizhong Du for his involvement in the original study design.
Funding: All phases of this study were supported by Scientific Research Fund of Zhejiang Provincial Education Department (Y200908620) and Zhejiang province innovation team for early screening and intervention of birth defects (N20110661-02).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Prior PF, Maynard DE, Sheaff PC, et al. Monitoring cerebral function: clinical experience with new device for continuous recording of electrical activity of brain. Br Med J 1971;2:736-8. [PubMed]
- Bjerre I, Hellström-Westas L, Rosén I, et al. Monitoring of cerebral function after severe asphyxia in infancy. Arch Dis Child 1983;58:997-1002. [PubMed]
- Toet MC, Hellström-Westas L, Groenendaal F, et al. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 1999;81:F19-23. [PubMed]
- ter Horst HJ, Sommer C, Bergman KA, et al. Prognostic significance of amplitude-integrated EEG during the first 72 hours after birth in severely asphyxiated neonates. Pediatr Res 2004;55:1026-33. [PubMed]
- van Rooij LG, Toet MC, van Huffelen AC, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics 2010;125:e358-66. [PubMed]
- Shany E, Khvatskin S, Golan A, et al. Amplitude-integrated electroencephalography: a tool for monitoring silent seizures in neonates. Pediatr Neurol 2006;34:194-9. [PubMed]
- Toet MC, Groenendaal F, Osredkar D, et al. Postneonatal epilepsy following amplitude-integrated EEG-detected neonatal seizures. Pediatr Neurol 2005;32:241-7. [PubMed]
- Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663-70. [PubMed]
- Zhang D, Liu Y, Hou X, et al. Reference values for amplitude-integrated EEGs in infants from preterm to 3.5 months of age. Pediatrics 2011;127:e1280-7. [PubMed]
- Agardh CD, Rosén I. Neurophysiological recovery after hypoglycemic coma in the rat: correlation with cerebral metabolism. J Cereb Blood Flow Metab 1983;3:78-85. [PubMed]
- Harris DL, Weston PJ, Williams CE, et al. Cot-side electroencephalography monitoring is not clinically useful in the detection of mild neonatal hypoglycemia. J Pediatr 2011;159:755-760.e1.
- Ter Horst HJ, Bos AF, Duijvendijk J, et al. Moderate unconjugated hyperbilirubinemia causes a transient but delayed suppression of amplitude-integrated electroencephalographic activity in preterm infants. Neonatology 2012;102:120-5. [PubMed]
- ter Horst HJ, van Olffen M, Remmelts HJ, et al. The prognostic value of amplitude integrated EEG in neonatal sepsis and/or meningitis. Acta Paediatr 2010;99:194-200. [PubMed]
- Olischar M, Shany E, Aygün C, et al. Amplitude-integrated electroencephalography in newborns with inborn errors of metabolism. Neonatology 2012;102:203-11. [PubMed]
- MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ 1999;319:1054-9. [PubMed]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696-705. [PubMed]
- Burns CM, Rutherford MA, Boardman JP, et al. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 2008;122:65-74. [PubMed]
- Mao J, Chen LY, Fu JH, et al. Clinical evaluation of neonatal hypoglycemic brain injury demonstrated by serial MRIs. Zhongguo Dang Dai Er Ke Za Zhi 2008;10:115-20. [PubMed]
- Gleason CA, Devaskar S. eds. Avery’s Diseases of the Newborn. 9th edition. Philadelphia, PA: Saunders Elsevier, 2000.
- Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr 2002;140:396-403. [PubMed]
- Maynard D, Prior PF, Scott DF. Device for continuous monitoring of cerebral activity in resuscitated patients. Br Med J 1969;4:545-6. [PubMed]
- Hellström-Westas L, Rosén I. Continuous brain-function monitoring: state of the art in clinical practice. Semin Fetal Neonatal Med 2006;11:503-11. [PubMed]
- Bayley N. Bayley Scales of Infant Development, 2nd ed. San Antonio: The Psychological Corporation, 1993.
- Burdjalov VF, Baumgart S, Spitzer AR. Cerebral function monitoring: a new scoring system for the evaluation of brain maturation in neonates. Pediatrics 2003;112:855-61. [PubMed]
- Osredkar D, Toet MC, van Rooij LG, et al. Sleep-wake cycling on amplitude-integrated electroencephalography in term newborns with hypoxic-ischemic encephalopathy. Pediatrics 2005;115:327-32. [PubMed]
- El-Dib M, Chang T, Tsuchida TN, et al. Amplitude-integrated electroencephalography in neonates. Pediatr Neurol 2009;41:315-26. [PubMed]
- al Naqeeb N, Edwards AD, Cowan FM, et al. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics 1999;103:1263-71. [PubMed]
- Shany E, Goldstein E, Khvatskin S, et al. Predictive value of amplitude-integrated electroencephalography pattern and voltage in asphyxiated term infants. Pediatr Neurol 2006;35:335-42. [PubMed]
- McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 2000;55:506-13. [PubMed]
- Liu X, Muller RU, Huang LT, et al. Seizure-induced changes in place cell physiology: relationship to spatial memory. J Neurosci 2003;23:11505-15. [PubMed]
- Liu Z, Yang Y, Silveira DC, et al. Consequences of recurrent seizures during early brain development. Neuroscience 1999;92:1443-54. [PubMed]


