Association of folate metabolism genes MTHFR and MTRR with multiple complex congenital malformation risk in Chinese population of Shanxi
Introduction
Birth defects are structural malformations present in a baby at or before birth affecting multiple different organs. Data from the Chinese Birth Defects Monitoring Program (CBDMP) indicated that neural tube defects (NTD) rate at birth in the whole of China is 2.7‰, but is high in Shanxi Province about 10.6‰ (1). The most common birth defects are congenital heart defects (CHD), NTD and craniofacial malformations which include cleft lip with or without cleft palate (CL/P) and cleft palate only (CPO) in USA (2). While in Chinese Shanxi the most common birth defects are anencephaly (10‰), congenital heart diseases (7.32‰) and spina bifida (6.39‰) (3). And it was reported that many birth defects cases presented as multiple congenital malformations beside single congenital malformations (4,5). The etiology of birth defects has been widely discussed but is not yet fully clarified.
Numerous studies suggest that the genetic and environmental factors are involved in birth defects information and it is reported that folate plays a pivotal role in normal embryonic development (2). Pregnant experimental animals subjected to folate deprivation often fail to deliver normal pups at term, instead of giving birth to pups with multiple malformations (6,7). Epidemiological studies have demonstrated that the benefit of folic acid supplementation in preventing NTD and other congenital abnormalities.
Folate, which acts as a one-carbon donor involved in both the synthesis of nucleotides and methyl transfer reactions, is important for methylation of DNA, protein and lipids. Epidemiological studies provide evidence that among environmental factors, folate status plays a key role in birth defect. Polymorphic variants of genes in the folate and homocysteine pathways have well-associated with the risk of birth defects, including common variants rs1801133 (c.677C>T) of MTHFR (5, 10-methylenetetrahydrofolate reductase) and rs1801394 (c.66A>G) of MTRR (methionine synthase reductase). It is reported that rs1801133 was associated with the risk of CL/P, NTD and CHD (8-12), while rs1801394 was associated with the risk of CHD and NTD (13-15). Embryogenesis is a restrict hierarchy developing process and birth defects occur at the earlier stage. CL/P, NTD and CHD were developed from different germ layers. So we want to know that whether the two folate pathway related polymorphic variants are having? A common risk fact related to birth defects and which germ layer the two folate pathway associated polymorphic variants are related to.
Here we gathered 420 normal controls and 250 birth defects. Per case presented 1-8 types of congenital malformations and classified the phenotypes into three germ layers-developed. By genotyping of rs1801394 and rs1801133, we further identified the associations of genetic variants with the risks of birth defects and germ layers related. Our results suggest that rs1801394 and rs1801133 were associated with multiple phenotypes. And genetic variants of rs1801133 and rs1801394 were associated with the risk of ectoderm- and endoderm-developed malformations, but only the variant of rs1801394 was associated with the risk of mesoderm-developed malformations. These results indicate that birth defects are associated with the function of folate metabolism and homocysteine pathways.
Materials and methods
Subjects
Stillborn birth defects case subjects were obtained from Shanxi Province of Northern China (16). This study was approved by the Committee of Medical Ethic in the Capital Institute of Pediatrics, Beijing, China; and written informed consent was received by the adult subjects and written informed consent from the parents on behalf of the minors. The enrolled pregnant women were diagnosed by local trained clinicians and ultrawave, and then registered in a database. The surgical details were described in our previous paper (17). In the present study 250 birth defects subjects were employed who were diagnosed with CHD, NTD and craniofacial malformations covering gestational ages more than 13 weeks. A total of 420 unrelated region-matched healthy subjects were recruited as normal control.
SNP identification and genotyping
Genomic DNA was isolated from muscle tissue and extracted with the Blood and Tissue DNA Kit (QIAGEN, Dusseldorf, Germany) according to manufacturer’s instructions. The concentration and purity of DNA were determined by absorbance at 260 and 280 nm.
SNPs were genotyped using the SNaPshot analysis (ABI). In brief, the genomic DNA was individually amplified by using the primers listed in Table 1, After SAP and Exo I purification, obtained purified template which include target SNP site. According to manufacturer’s instructions, extent primer (as shown in Table 1) and SNaPshot Multiplex were added into the template and the thermal cycling reaction was done. Then the genotyping samples were run on ABI 3730 automated sequencer and analyzed by Peakscan software.

Full table
Statistical analysis
Hardy-Weinberg equilibrium (HWE) in control was tested by χ2 test and the P values are larger than 0.05. To evaluate the associations between genotypes and case risks, OR and 95% CIs were calculated by unconditional logistic regression analysis with adjustment for age using the SNP Stats website (http://bioinfo.iconcologia.net/snpstats/start.htm). Each SNP was evaluated under four genetic models: a codominant model, a dominant model, a recessive model and a log additive model. All statistical tests were two-tailed, with P<0.05 regards as statistically significance, and performed by SPSS 15.0 software (SPSS, Chicago, IL, USA).
Results
Description and classification of disease phenotypes
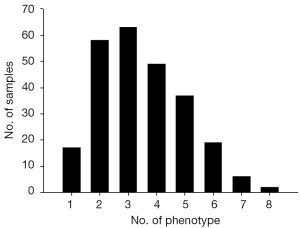
To characterized the present group of birth defect cases, we described the phenotypes of birth defects and complications according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) Version for 2010 (Figure 1A). Then, the presented phenotypes were sorted into ectoderm-, mesoderm- and endoderm-developed (Figure 1B). Multiple birth defects are defined as two or more unrelated major structural malformations that cannot be explained by an underlying syndrome or sequence (4,18). Figure 2 presented the sample characters in our cases, it shows that multiple birth defects were chose.
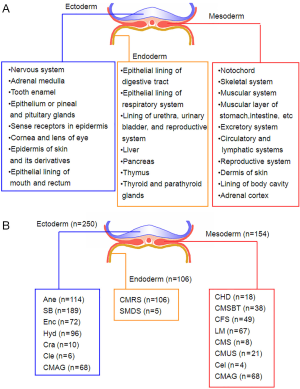
Associations of SNPs with risks of various disease phenotypes in cases
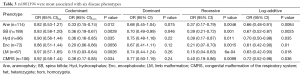
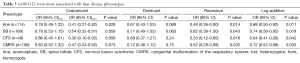
To identify whether the SNPs rs1801394 and rs1801133 were associated with the risk of birth defects and the germ layers, SNaPshot method was used to genotype both of SNPs sites. Considering the presentation of diseases, some congenital malformations were given up to be analyzed if the case amounts were less than 30. The results indicated that the odds ratios (OR) and 95% confidence intervals (CIs) of recessive homozygote of rs1801394 were 0.33 (0.15-0.74) in anencephaly (Ane), 0.36 (0.19-0.67) in spina bifida (SB), 0.36 (0.16-0.85) in congenital hydrocephalus (Hyd), 0.20 (0.06-0.66) in encephalocele (Enc), 0.15 (0.03-0.64) in Limb malformation (LM) and 0.38 (0.17-0.85) in congenital malformation of the respiratory system (CMRS) (Tables 2,3). The values of recessive homozygote of rs1801133 were 0.41 (0.21-0.82) in Ane, 0.54 (0.32-0.91) in SB, 0.30 (0.10-0.92) in cervical fusion syndrome (CFS) and 0.47 (0.24-0.92) in CMRS (Tables 4,5). The data displayed that rs1801394 is associated with the risks of six types of disease phenotypes while rs1801133 is associated with of four types.

Full table
Full table

Full table
Full table
Association of SNPs with risks of germ layer-developed tissue defects
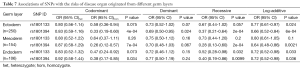
Since rs1801394 and rs1801133 are associated with the risks of multiple phenotypes in the same cases population, this promotes us to consider whether the etiologies of multiple malformations presented on one population are resulted from a common developmental defects. Therefore, the associations of SNPs with the risks of three germ layers-developed tissues defects (as shown in Figure 1B) were analyzed. The results indicated that in ectoderm- and endoderm-developed groups, the OR and 95% CI of recessive homozygote of rs1801133 were 0.58 (0.36-0.94) and 0.47 (0.24-0.92); and of rs1801394 were individually 0.33 (0.19-0.60) and 0.38 (0.17-0.85) (Tables 6,7). However, in mesoderm-developed group, only the OR and 95% CI of rs1801394 were 0.26 (0.12-0.57) (Tables 6,7).

Full table
Full table
Discussion
The present study indicated that genetic variants of MTRR and MTHFR were associated with risks of multiple disease phenotypes presented on Chinese North population. Further, they conferred protective factors of ectoderm- and endoderm-developed tissue malformations. However, only MTRR genetic variation was a protective factor of mesoderm-developed tissue malformations.
Our data presented an implication that causation of birth defect and complications in humans could be resulted from the nutrition gene factors in mammals, some previous studies supported this viewpoint. During gastrulation the visceral endoderm defects were formed because of the mutation of the Slc40a1 gene encoding the iron transporter ferroportin1; and further the mutant embryo were developmentally delayed and exhibited exencephaly, microphthalmia and generalized edema (19), Moreover, Slc40a1 knockout mice results in embryonic lethality at E7.5 (20). Similarly, the failure of distal visceral endoderm migration and primitive streak formation were brought on deletion of mouse prickle1 gene, one of the core components of planar cell polarity signaling (21). Importantly, in humans a homozygous mutation PRICKLE1 caused an autosomal recessive progressive myoclonus epilepsy-ataxia syndrome, their further experiment validated that the mutation could reduce the effect of overexpression of wild type prickle1 and decreased gastrulation defects (22). Taken together, these lines of evidence demonstrate that in early stages of embryogenesis, mild mutation may result in the embryonic defects. In addition, the roles of both genes in visceral endoderm developing may be a clue for the present study in which ectoderm-developed defect seems to has a closer association to endoderm- than mesoderm-developed defect, because during gastrulation visceral endoderm and yolk sac provide for the nutritional support (including the uptake of folate) and exchange of waste products between the maternal circulation and the developing embryo (23).
In the present study the genetic variants rs1801394 and rs1801133 as important biological markers hint us the etiologies of NTDs and other malformations in various stages of development. During the embryogenesis, MTRR is transcriptionally expressed during metaphase I of oocyte to blastocyst in the pre-implantation development (24), and distributed in the neural tube and other tissues (25). MTRR knockout results in embryonic lethality and a hypomorph, with reduced MTRR activity by gene trap technology, adversely impacts reproductive outcomes and birth defects (25,26). The variant rs1801394 locus is in flavordoxin domain of MTRR gene and the mutation affects the affinity of MTRR to MTR (27). These indicated that rs1801394 is a functional genetic variant and potentially affects the early embryogenesis. MTHFR is expressed from metaphase I oocyte to senescence. Mild MTHFR deficiency caused by c.677C→T encodes a thermolabile enzyme with reduced activity (28). MTHFR knockout significantly decreased S-adenosylmethionine levels and increased S-adenosylhomocysteine levels with global DNA hypomethylation, and further show developmental retardation with cerebellar pathology and abnormal lipid deposition (29), which are consistent with our previous studies that in the NTDs population in Shanxi Province area, global DNA are hypomethylated (17,30,31). Taken together, these hint us that MTRR and MTHFR function spanning the embryogenesis, and their functional genetic variants potentially impact the embryo development at the early stage of embryogenesis, hence, result in complicated disease phenotypes.
In summary, our present study first reports an overall view about a subset of birth defect cases in Chinese northern population of Shanxi. And upon their complicated phenotypes, we use two genetic variants of MTRR and MTHFR, both of which play roles in early embryogenesis, to discover the probability of common tissue pathogenesis. The implication could be a reminder for our consideration about the etiology of birth defects, furthermore, the present study offers an experimental support for the supplementary of folic acid in the early stage of embryogenesis.
Acknowledgements
We are grateful to all participating hospitals for their assistance in sample collection and recording of clinical information. We thank all of the women who participated for their cooperation.
Funding: This work was supported by grants from National Natural Science Foundation of China (No. 81300489).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen G, Pei LJ, Huang J, et al. Unusual patterns of neural tube defects in a high risk region of northern China. Biomed Environ Sci 2009;22:340-4. [PubMed]
- Zhu H, Kartiko S, Finnell RH. Importance of gene-environment interactions in the etiology of selected birth defects. Clin Genet 2009;75:409-23. [PubMed]
- Zheng XY, Song XM, Chen G, et al. Epidemiology of birth defects in high-prevalence areas of China. Zhonghua Liu Xing Bing Xue Za Zhi 2007;28:5-9. [PubMed]
- Ooki S. Maternal age and birth defects after the use of assisted reproductive technology in Japan, 2004-2010. Int J Womens Health 2013;5:65-77. [PubMed]
- North K, McCredie J. Neurotomes and birth defects: a neuroanatomic method of interpretation of multiple congenital malformations. Am J Med Genet Suppl 1987;3:29-42. [PubMed]
- Nelson MM, Asling CW, Evans HM. Production of multiple congenital abnormalities in young by maternal pteroylglutamic acid deficiency during gestation. J Nutr 1952;48:61-79. [PubMed]
- Nelson MM, Baird CD, Wright HV, et al. Multiple congenital abnormalities in the rat resulting from riboflavin deficiency induced by the antimetabolite galactoflavin. J Nutr 1956;58:125-34. [PubMed]
- Huang J, Mei J, Jiang L, et al. MTHFR rs1801133 C>T polymorphism is associated with an increased risk of tetralogy of Fallot. Biomed Rep 2014;2:172-6. [PubMed]
- Yu X, Liu J, Zhu H, et al. Synergistic association of DNA repair relevant gene polymorphisms with the risk of coronary artery disease in northeastern Han Chinese. Thromb Res 2014;133:229-34. [PubMed]
- Blanton SH, Henry RR, Yuan Q, et al. Folate pathway and nonsyndromic cleft lip and palate. Birth Defects Res A Clin Mol Teratol 2011;91:50-60. [PubMed]
- Pangilinan F, Molloy AM, Mills JL, et al. Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Med Genet 2012;13:62. [PubMed]
- Etheredge AJ, Finnell RH, Carmichael SL, et al. Maternal and infant gene-folate interactions and the risk of neural tube defects. Am J Med Genet A 2012;158A:2439-46. [PubMed]
- Cai B, Zhang T, Zhong R, et al. Genetic variant in MTRR, but not MTR, is associated with risk of congenital heart disease: an integrated meta-analysis. PLoS One 2014;9:e89609. [PubMed]
- Shaw GM, Lu W, Zhu H, et al. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med Genet 2009;10:49. [PubMed]
- Relton CL, Wilding CS, Pearce MS, et al. Gene-gene interaction in folate-related genes and risk of neural tube defects in a UK population. J Med Genet 2004;41:256-60. [PubMed]
- Gu X, Lin L, Zheng X, et al. High prevalence of NTDs in Shanxi Province: a combined epidemiological approach. Birth Defects Res A Clin Mol Teratol 2007;79:702-7. [PubMed]
- Wang L, Wang F, Guan J, et al. Relation between hypomethylation of long interspersed nucleotide elements and risk of neural tube defects. Am J Clin Nutr 2010;91:1359-67. [PubMed]
- Garne E, Dolk H, Loane M, et al. Paper 5: Surveillance of multiple congenital anomalies: implementation of a computer algorithm in European registers for classification of cases. Birth Defects Res A Clin Mol Teratol 2011;91 Suppl 1:S44-50. [PubMed]
- Mao J, McKean DM, Warrier S, et al. The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure. Development 2010;137:3079-88. [PubMed]
- Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 2005;1:191-200. [PubMed]
- Tao H, Suzuki M, Kiyonari H, et al. Mouse prickle1, the homolog of a PCP gene, is essential for epiblast apical-basal polarity. Proc Natl Acad Sci U S A 2009;106:14426-31. [PubMed]
- Bassuk AG, Wallace RH, Buhr A, et al. A homozygous mutation in human PRICKLE1 causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am J Hum Genet 2008;83:572-81. [PubMed]
- Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol 1999;43:183-205. [PubMed]
- Zhang P, Zucchelli M, Bruce S, et al. Transcriptome profiling of human pre-implantation development. PLoS One 2009;4:e7844. [PubMed]
- Elmore CL, Wu X, Leclerc D, et al. Metabolic derangement of methionine and folate metabolism in mice deficient in methionine synthase reductase. Mol Genet Metab 2007;91:85-97. [PubMed]
- Deng L, Elmore CL, Lawrance AK, et al. Methionine synthase reductase deficiency results in adverse reproductive outcomes and congenital heart defects in mice. Mol Genet Metab 2008;94:336-42. [PubMed]
- Olteanu H, Wolthers KR, Munro AW, et al. Kinetic and thermodynamic characterization of the common polymorphic variants of human methionine synthase reductase. Biochemistry 2004;43:1988-97. [PubMed]
- Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10:111-3. [PubMed]
- Chen Z, Karaplis AC, Ackerman SL, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet 2001;10:433-43. [PubMed]
- Chen X, Guo J, Lei Y, et al. Global DNA hypomethylation is associated with NTD-affected pregnancy: A case-control study. Birth Defects Res A Clin Mol Teratol 2010;88:575-81. [PubMed]
- Chang H, Zhang T, Zhang Z, et al. Tissue-specific distribution of aberrant DNA methylation associated with maternal low-folate status in human neural tube defects. J Nutr Biochem 2011;22:1172-7. [PubMed]


