Hemispherectomy in the treatment of seizures: a review
History and evolution of technique
In 1928, one of the pioneers of neurological surgery, Walter Dandy, described a series of five patients who underwent removal of their right cerebral hemispheres for cerebral gliomas (1). This represented a radical attempt at achieving cure of what at the time was an incurable disease. He described a technique involving ligation of the anterior and middle cerebral arteries and subsequent en bloc removal of the cerebral hemisphere in a seemingly crude fashion (“…first to section the frontal lobe, which requires only a sweep of the scalpel…entering the anterior horn of the lateral ventricle with the index finger, to use this as a lever…with scissors or the edge of a spatula, the internal capsule is divided flush with the ventricle”). Three of the five patients died within 3 months of surgery (one immediately after due to hemorrhage).
W. James Gardner, a Cleveland neurosurgeon reported an additional three cases of right cerebral hemisphere removal for glioma in 1933 (2). Two of these patients died within 36 hours of surgery of “hyperthermia”. A third patient, however, was seizure free, hemiparetic but cognitively well and ambulatory without evidence of recurrence 2 years after surgery. This was the first report of a patient remaining ambulatory after a hemispherectomy (which was erroneously attributed to preservation of the basal ganglia). It demonstrated that a reasonable quality of life could be preserved after right hemisphere removal. While this radical approach to glioma surgery ultimately proved inappropriate, it served as a blueprint for subsequent hemispherectomy surgery for epilepsy.
The first hemispherectomy performed specifically for epilepsy was reported by the Canadian neurosurgeon K.G. McKenzie at the American Medical Association meeting in Chicago in 1938 (3). The patient presented to Toronto General Hospital at the age of 16, with a prior history of a head injury at the age of three weeks with subsequent left hemiplegia and epilepsy. She underwent a right hemispherectomy which successfully alleviated her seizures. The first series of patients undergoing hemispherectomy surgery for epilepsy was reported by Krynauw RA in 1950 (4). He described 12 children with “infantile hemiplegia” who underwent hemispherectomies. Interestingly, two of the patients did not have epilepsy; Krynauw stated, “I have come to consider mental deviations in the absence of epileptic phenomena, and also epileptic manifestations in the absence of definite mental or behavior disturbances, as adequate indications for surgery”. Of the ten patients who did have seizures, one died immediately after surgery without explanation. The remaining nine patients however did quite well with resolution of seizures and well documented preservation of some useful ipsilateral motor control and ambulatory status in most cases.
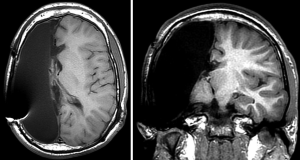
Krynauw’s successes lead to a period of enthusiasm for what is now termed to be the anatomic hemispherectomy (AH)—the complete removal of the cerebral hemisphere with or without preservation of the basal ganglia and thalamus (Figure 1). Over time it became apparent that a significant portion of patients were deteriorating on a delayed basis. In 1965, Ulrich et al. first reported superficial cerebral hemosiderosis as a late complication of hemispherectomy surgery (5). Superficial cerebral hemosiderosis is characterized by diffuse iron depositions within the meninges, ependymal, and cerebral cortex as a consequence of repeated hemorrhage into the large subdural resection cavity. In 1966, Oppenheimer and Griffith reported on their series of 17 AH patients, four of whom died on a delayed basis after several years of good health. In three patients available for autopsy, they noted ventricular dilation consistent with hydrocephalus as well as superficial hemosiderosis which they postulated to have been the cause of the hydrocephalus. As the use of ventricular shunting became more prevalent, some of the morbidity caused by the associated hydrocephalus was mitigated, but repetitive hemorrhage was still a frequent problem. In 1973 (6), Rasmussen reported results from the Montreal Neurological Institute (MNI), where the incidence of superficial hemosiderosis was 33% in AH patients. Rasmussen made the key observation that in patients with “subtotal” hemispherectomies (multilobar resections without complete AH), hemosiderosis was not seen. By 1968, MNI surgeons had begun to intentionally leave either frontal or occipital pole brain behind while accepting a higher rate of persistent seizures, to avoid this complication (6).
The problem of superficial cerebral hemosiderosis dampened enthusiasm for hemispherectomy surgery and led to the first branch point in the evolution of hemispherectomy techniques. Some centers continued to perform anatomic hemispherectomies, albeit with modifications. In 1983, Adams reported on a small series of patients undergoing AH with plication of the dura to the falx cerebri and tentorium cerebelli with plugging of the foramen of Monro to obliterate the subdural resection cavity and its communication with the rest of the ventricular system (7). At UCLA, Peacock addressed the problem by placing resection cavity drains, then electively shunting most of his AH patients to divert blood products and prophylactically address hydrocephalus (8). Others moved away from AH techniques. One tactic developed to avoid superficial hemosiderosis was the development of hemidecortication—the practice of removing all of the cortex while minimizing exposure of the ventricular system by maintaining surrounding white matter (9,10). (It should be noted that older literature employs the term “hemidecortication” interchangeably with what would now be called “AH”). Rasmussen refined his technique of intentionally leaving brain tissue behind by disconnecting such tissue from the corpus callosum and upper brainstem, resulting in a “functional complete but anatomical subtotal hemispherectomy” or functional hemispherectomy (FH) (11). The Rasmussen FH involved removal of the temporal lobe and a central portion of frontoparietal brain. The access this provided was used to perform frontal and posterior disconnections between the retained brain and midbrain as well as a complete corpus callosotomy. All of these modifications appeared to prevent superficial hemosiderosis, leading to renewed acceptance of hemispherectomy surgery as an appropriate surgical option for select cases.
Hemispherotomy is a direct descendant of Rasmussen’s FH, utilizing the same principles of leaving living vascularized brain behind that is disconnected from healthy brain. The distinction between hemispherotomy and FH is a relatively small one related to the more minimalistic amount of brain physically removed with hemispherotomy techniques. Hemispherotomy techniques were introduced in the 1990’s, by Delalande (12), Villemure (13,14), and Schramm (15-17), each with their own solution to the achieving the disconnections required to attain complete functional disconnection of the hemisphere. Cook et al. (18) described a modified lateral hemispherotomy which involves sacrifice of the middle cerebral artery with removal of a central block of opercular tissue. Bahuleyan et al. (19) demonstrated the feasibility of a purely endoscopic transventricular hemispherectomy on cadaver brains as a proof of concept. Hemispherotomy techniques are continually refined and predominate at most epilepsy centers in the 21st century.
Patient population
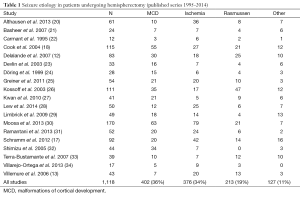
Table 1 shows a breakdown of seizure etiology from the larger published series of the last 20 years. By aggregating case counts, one can get a sense of the frequency of pathology leading to hemispherectomy surgery. The vast majority of patients are pediatric as most of the pathology and medically refractory epilepsy are present early in life. Candidates for potential epilepsy surgery undergo an extensive workup to confirm as best as possible that: (I) the seizures are emanating exclusively from one hemisphere only; and (II) a smaller resection sparing functional brain would not adequately address seizures. The typical preoperative evaluation includes: a detailed neuropsychological evaluation, long-term video electroencephalography (EEG), and MRI. Other studies often but now always utilized include: functional MRI, Wada testing, positron emission tomography (PET), and magnetoencephalography (MEG). Potential cases are discussed at multidisciplinary conferences that include neurosurgeons, epileptologists, neuroradiologists, and neuropsychologists.
Full table
Malformations of cortical development (MCD)
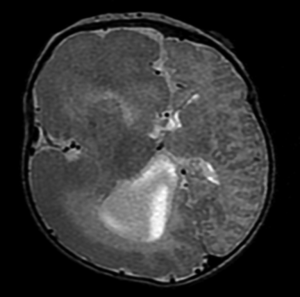
The term MCD, also known as cortical dysplasias, cortical dysgenesis, or neuronal migration disorders, encompasses a wide variety of developmental brain anomalies often associated with developmental delay, neurological deficits, and epilepsy. There are a wide variety of abnormalities that fall under the rubric of MCD, and can be categorized based on histopathology, imaging, genetics, as well as clinical and electrographic features (35-37). Patients with broad unilateral MCD comprise a large portion of hemispherectomy candidates. Hemimegalencephaly is such a condition, characterized by unilateral enlargement all or most of a cerebral hemisphere, with focal or diffuse microscopic cortical abnormalities (Figure 2). Patients with hemimegalencephaly commonly have medically refractory epilepsy, often at a very young age, which is responsive to hemispherectomy surgery. Some patients with MCD have large dysplastic epileptogenic networks that require multilobar resection or hemispherectomy to effectively mitigate seizures. A fair number of patients with MCD undergoing hemispherectomy surgery will have already had prior more conservative resections that failed to adequately stop seizures.
Ischemia
In most hemispherectomy series, patients with large hemispheric infarcts represent a significant portion of patients (Table 1). Most of these patients had their strokes during the perinatal period. A recent prospective study of 46 infants with perinatal arterial strokes showed that 24% had at least one seizure, and 13% developed epilepsy within a mean follow-up period of 31 months. Larger stroke size was associated with a 6-fold increase in seizure risk (38). When the seizures prove to be refractory to medical management, these patients are excellent candidates for hemispherectomy surgery as they typically already have hemiparesis and hemianopsia, with language development in the contralateral healthy hemisphere. Thus they typically do not suffer any further decline in function with hemispherectomy. Patients with perinatal strokes or more likely than MCD to have a completely normal contralateral hemisphere as well, theoretically decreasing the chance of persistent seizures post-hemispherectomy.
Rasmussen’s encephalitis (RE)
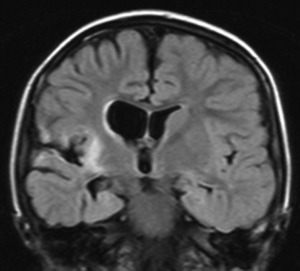
RE is a rare condition that comprises a significant portion of population of patients who undergo hemispherectomy surgery, ranging from 0% (32) to 42% (39) of hemispherectomy cases reported. As the name implies, RE is an inflammatory process, albeit one that is poorly understood. The hallmark of RE is progressive unilateral hemispheric dysfunction characterized by inflammatory changes on imaging and histopathology. The etiology is unknown with most cases occurring sporadically with some reports of RE onset after head injury or infection (40). There is evidence of an autoimmune pathophysiological mechanism in RE, and autoantibodies to the NMDA glutamate receptor type-3 (GluR3) have been found in association with RE and other severe forms of epilepsy (41,42). RE typically (but not exclusively) affects children, who present with progressively worsening unilateral focal-onset seizures, classically epilepsia partialis continua (EPC), followed by a progressive decline in unilateral hemispheric function manifesting as hemiparesis. Language function can also deteriorate if the dominant hemisphere is affected. MRI (Figure 3) demonstrates initially areas of inflammation, followed by progressive atrophy (43). Immunomodulatory therapy with high dose corticosteroids, intravenous immunoglobulin (IVIG), calcineurin-inhibitors or plasma exchange have been attempted to slow the progression of the disease with mixed results (44-48). Hemispherectomy surgery remains the only treatment shown to definitively halt the progression of the disease.
Other pathology
Sturge-Weber syndrome (SWS, also known as encephalotrigeminal angiomatosis) is a neurocutaneous disorder characterized by facial and leptomeningeal angiomas (Figure 4). The leptomeningeal vascular anomalies of SWS are typically unilateral and can result in gradual ischemic changes, including atrophy, calcification, and gliosis with associated neurological decline and epilepsy. Epilepsy develops in 75-80% of patients with SWS (49). Due to the often hemispheric involvement of the disease, hemispherectomy surgery is utilized for seizure control in select cases, typically when there is already prior loss of hemispheric motor function from prior ischemia (50).
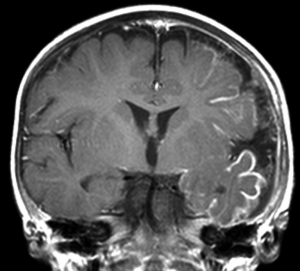
Hemiconvulsion-hemiplegia-epilepsy (HHE) syndrome is a rare, poorly understood disorder affecting young children, typically under the age of 4 years. The hallmarks of HHE include a febrile illness with associated unilateral-onset seizures, hemispheric edema not confined to a vascular distribution, with subsequent difficult to control epilepsy and hemispheric dysfunction with atrophy (51). As with many hemispherectomy candidates, the patients have pre-existing hemiplegia at the time of surgery.
Head trauma is a relatively rare indication for hemispherectomy surgery. Head trauma only rarely leads to intractable epilepsy and in most cases the damage is bilateral, thus patients are not likely to be acceptable candidates due to either bilateral seizure onset, or bilateral existing cerebral damage making hemispherectomy surgery unacceptable with regards to functional loss. Although one recent hemispherectomy series included a patient with a malignant tumor status post local radiation therapy and subsequent intractable epilepsy leading to hemispherectomy (28), tumor patients rarely develop a need for such radical surgery for seizure control.
Outcomes
Seizure freedom
Hemispherectomy surgery is probably the most successful form of epilepsy surgery available in terms of achieving seizure freedom. Rates of seizure freedom (Engel I) in published series over the past decade range from 54% to 90% (17,18,20,21,27,28,32,33,52-54). Most patients who do not achieve seizure freedom enjoy some improvement in their seizure burden (Engel II or III). The reason for failure is not always apparent for an individual case. Reasons to continue to seize following hemispherectomy surgery include: (I) unrecognized seizures emanating from the contralateral hemisphere (i.e., misdiagnosis); (II) failure to adequately disconnect or remove the entire hemisphere (i.e., technical error); or (III) the development of a new seizure focus in the contralateral hemisphere (progression of disease). It should be also noted that hemispherectomy surgery may be offered in cases where there is bilateral disease with the hope that antiepileptiform medication can control the contralateral hemisphere seizures. It is also offered at times as a purely palliative procedure for severe cases with bilateral seizure onset when one side predominates (28,55,56).
The study of hemispherectomy surgery outcome is made difficult by the relative infrequency of the procedure. Nearly all published data are retrospective single-center experiences. The largest published series to date by Moosa et al. includes a total of only 186 patients. Only 12 other series include over 40 patients (12,13,17,18,20,25,27-29,31,39,57). From a statistical standpoint, it is difficult to determine what variables have significant influence over outcome. Only six studies (20,23,31,39,54,57) have identified preoperative factors that correlated with seizure outcome with demonstrable statistical significance. Two studies demonstrated that bilateral imaging abnormalities correlated with worsened seizure outcome, a finding that makes intuitive sense (54,57). Two studies identified younger age as being predictive of improved seizure outcome (20,31), a finding not duplicated in the two largest published series (53,54). Two studies demonstrated statistically significant correlations between seizure etiology and seizure outcome, with developmental pathology such as MCD (and hemimegalencephaly in particular) associated with a lower rate of seizure freedom (23,39). This finding has not been reproduced in any other series, which may reflect a lack of power in these individual studies rather than a lack of effect. MCD is more likely to be associated with bihemispheric structural abnormalities compared to most other conditions leading to hemispherectomy. Hemimegalencephaly also poses a greater surgical challenge (atypical anatomy, enlarged brain, small or aberrant ventricular system) and possibly a higher rate of technically inadequate resections/disconnections.
There is not a consensus regarding optimal surgical technique. Most centers have migrated to one procedure of choice and it is difficult to compare techniques by comparing outcomes from different centers without being able to control for patient selection. Only one single institution, retrospective, non-randomized study found a difference in seizure outcome between two techniques. Kwan et al. reported the Toronto experience of discontinuing hemidecortications in favor of periinsular hemispherotomies with the latter yielding a higher rate of Engel I/II outcomes (85% vs. 48%, P<0.02). To answer the question of technique superiority, a randomized prospective trial would be required (which is not likely to happen). Nevertheless, the trend away from anatomic hemispherectomies towards hemispherotomies at most experienced centers is likely an indication of the superiority of hemispherotomy techniques with regards to complications if not seizure efficacy as well.
Functional outcome
Some more recent series on hemispherectomy outcome include preoperative and postoperative neuropsychological measures (20,28,53,58-61). Although individual patient outcomes may vary, with occasional significant declines or improvement, the overall cognitive function is stable at the group-level in these studies. Lower preoperative intelligence (20), nonhemimegalencephalic MCD (53), absence of contralateral MRI abnormalities (57), older age at seizure onset (62), shorter duration of seizures (62), and postoperative seizure freedom (20) have been associated with postoperative cognitive improvement.
There is an expected contralateral homonymous hemianopsia after hemispherectomy. Motor deficits following hemispherectomy are variable. Most patients remain ambulatory (if they were preoperatively) (30). The typical hemiparesis is most pronounced with distal motor function of the upper and lower extremities. Patients with a history of perinatal stroke typicaly have more distal extremity function than those with other etiologies. Typically there is a loss of fine motor function in the hand and limited ankle dorsiflexion requiring an ankle-foot orthosis. de Bode et al. (63) demonstrated less distal extremity motor loss in patients with perinatal strokes compared to other epilepsy etiologies, irrespective of time of epilepsy onset or surgery. They also found that areas of increased ipsilateral activation with motor tasks on post-hemispherectomy functional MRI in areas not typically associated by motor function (cingulate, insula), and that the areas involved varied depending on etiology of epilepsy.
Complications
Hydrocephalus is a known adverse outcome of hemispherectomy surgery that has been reported in every large series. The incidence varies greatly between individual series, ranging from 9% to 81% (8,18,21,27,28,64-67). While the exact mechanism for development of hydrocephalus is unknown, it may be related to exposure of blood products and subsequent inflammatory processes to the intraventricular spaces (as seen with intraventricular hemorrhage of prematurity or meningitis). All current hemispherectomy techniques involve some entry to the ventricular system. Recently, 15 pediatric epilepsy centers pooled data on 690 children to examine the issue of post-hemispherectomy hydrocephalus (68). The overall incidence was 23%. Prior cranial surgery and the AH technique were identified as statistically significant risk factors for developing hydrocephalus.
As with any major intracranial procedure, hemispherectomy surgery is associated with a litany of minor complications such as infection, aseptic meningitis, and transient neurological deficits. Contralateral strokes and deaths are rarely found in modern era series (8,69). Although there is likely a reporting bias, the current risk of mortality from hemispherectomy surgery at experienced centers is likely below 1%.
There is a small literature on revision hemispherectomies. When a hemispherectomy unexpectedly fails to result in seizure freedom, the possibilities include an incomplete disconnection/resection or contralateral seizure generation. When the former is suspected, revision hemispherectomy surgery may be offered. This has been accomplished with selective disconnection or resection of suspected connected tissue or with conversion to an AH with seizure freedom rates ranging from 19% to 33% (68,70). Thus, there are patients who have technically incomplete hemispherectomies but the frequency of this complication has not been determined in any study. One reason for this lack of reporting is the difficulty of definitively assessing for residual connected tissue on postoperative imaging; there are no foolproof means of distinguishing living, functionally connected brain tissue from non-functional or unconnected tissue.
Future directions
Despite dramatic improvements in safety and efficacy, there are still significant complications and treatment failures. There undoubtedly will be continued refinement of techniques with better rates of seizure freedom and fewer complications. There is perhaps more room for improvement with regards to improving patient selection. Some if not most hemispherectomy failures are due to new or persistent seizures arising from the contralateral hemisphere. As the ability to identify the extents and limits of epileptogenic tissue, more targeted therapies can be utilized. Thus, some patients that are appropriate candidates for hemispherectomy currently may be better treated in the future with less morbid interventions that do not require the sacrifice of an entire cerebral hemisphere.
Conclusions
Hemispherectomy surgery has progressed from an extremely morbid procedure inappropriately applied to brain tumors to a set of refined procedures practiced routinely at major epilepsy centers around the world. While there are a variety of acceptable versions of hemispherectomy surgery with proven track records, there is a clear trend towards the abandonment of anatomic hemispherectomies for hemispherotomy techniques which involve more disconnection and less resection. With proper patient selection, outcomes are generally excellent with regards to seizure burden and serious complications. The majority of patients are ambulatory postoperatively with preservation of cognitive function. Future advancements are likely to come from improved selection of surgical candidacy and more targeted interventions that make sacrifice of a complete hemisphere unnecessary.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA 1928;90:823-5.
- Gardner WJ. Removal of the right cerebral hemisphere for infiltrating glioma. JAMA 1933;101:823-6.
- Williams DJ, Scott JW. The functional responses of the sympathetic nervous system of man following hemidecortication. J Neurol Psychiatry 1939;2:313-22. [PubMed]
- Krynauw RA. Infantile hemiplegia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry 1950;13:243-67. [PubMed]
- Ulrich J, Isler W, Vassalli L. The effect of repeated leptomeningeal hemorrhages on the nervous system (marginal siderosis of the central nervous system). Rev Neurol (Paris) 1965;112:466-71. [PubMed]
- Rasmussen T. Postoperative superficial hemosiderosis of the brain, its diagnosis, treatment and prevention. Trans Am Neurol Assoc 1973;98:133-7. [PubMed]
- Adams CB. Hemispherectomy--a modification. J Neurol Neurosurg Psychiatry 1983;46:617-9. [PubMed]
- Peacock WJ, Wehby-Grant MC, Shields WD, et al. Hemispherectomy for intractable seizures in children: a report of 58 cases. Childs Nerv Syst 1996;12:376-84. [PubMed]
- Hoffman HJ, Hendrick EB, Dennis M, et al. Hemispherectomy for Sturge-Weber syndrome. Childs Brain 1979;5:233-48. [PubMed]
- Ogunmekan AO, Hwang PA, Hoffman HJ. Sturge-Weber-Dimitri disease: role of hemispherectomy in prognosis. Can J Neurol Sci 1989;16:78-80. [PubMed]
- Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci 1983;10:71-8. [PubMed]
- Delalande O, Bulteau C, Dellatolas G, et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery 2007;60:ONS19-32; discussion ONS32.
- Villemure JG, Daniel RT. Peri-insular hemispherotomy in paediatric epilepsy. Childs Nerv Syst 2006;22:967-81. [PubMed]
- Villemure JG, Mascott CR. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery 1995;37:975-81. [PubMed]
- Schramm J, Behrens E, Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery 1995;36:509-15; discussion 515-6. [PubMed]
- Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery 2001;49:891-900; discussion 900-1. [PubMed]
- Schramm J, Kuczaty S, Sassen R, et al. Pediatric functional hemispherectomy: outcome in 92 patients. Acta Neurochir (Wien) 2012;154:2017-28. [PubMed]
- Cook SW, Nguyen ST, Hu B, et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg 2004;100:125-41. [PubMed]
- Bahuleyan B, Manjila S, Robinson S, et al. Minimally invasive endoscopic transventricular hemispherotomy for medically intractable epilepsy: a new approach and cadaveric demonstration. J Neurosurg Pediatr 2010;6:536-40. [PubMed]
- Althausen A, Gleissner U, Hoppe C, et al. Long-term outcome of hemispheric surgery at different ages in 61 epilepsy patients. J Neurol Neurosurg Psychiatry 2013;84:529-36. [PubMed]
- Basheer SN, Connolly MB, Lautzenhiser A, et al. Hemispheric surgery in children with refractory epilepsy: seizure outcome, complications, and adaptive function. Epilepsia 2007;48:133-40. [PubMed]
- Carmant L, Kramer U, Riviello JJ, et al. EEG prior to hemispherectomy: correlation with outcome and pathology. Electroencephalogr Clin Neurophysiol 1995;94:265-70. [PubMed]
- Devlin AM, Cross JH, Harkness W, et al. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain 2003;126:556-66. [PubMed]
- Döring S, Cross H, Boyd S, et al. The significance of bilateral EEG abnormalities before and after hemispherectomy in children with unilateral major hemisphere lesions. Epilepsy Res 1999;34:65-73. [PubMed]
- Greiner HM, Park YD, Holland K, et al. Scalp EEG does not predict hemispherectomy outcome. Seizure 2011;20:758-63. [PubMed]
- Kossoff EH, Vining EP, Pillas DJ, et al. Hemispherectomy for intractable unihemispheric epilepsy etiology vs outcome. Neurology 2003;61:887-90. [PubMed]
- Kwan A, Ng WH, Otsubo H, et al. Hemispherectomy for the control of intractable epilepsy in childhood: comparison of 2 surgical techniques in a single institution. Neurosurgery 2010;67:429-36. [PubMed]
- Lew SM, Koop JI, Mueller WM, et al. Fifty consecutive hemispherectomies: outcomes, evolution of technique, complications, and lessons learned. Neurosurgery 2014;74:182-94; discussion 195. [PubMed]
- Limbrick DD, Narayan P, Powers AK, et al. Hemispherotomy: efficacy and analysis of seizure recurrence. J Neurosurg Pediatr 2009;4:323-32. [PubMed]
- Moosa AN, Jehi L, Marashly A, et al. Long-term functional outcomes and their predictors after hemispherectomy in 115 children. Epilepsia 2013;54:1771-9. [PubMed]
- Ramantani G, Kadish NE, Brandt A, et al. Seizure control and developmental trajectories after hemispherotomy for refractory epilepsy in childhood and adolescence. Epilepsia 2013;54:1046-55. [PubMed]
- Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia 2005;46 Suppl 1:30-1. [PubMed]
- Terra-Bustamante VC, Inuzuka LM, Fernandes RM, et al. Outcome of hemispheric surgeries for refractory epilepsy in pediatric patients. Childs Nerv Syst 2007;23:321-6. [PubMed]
- Villarejo-Ortega F, García-Fernández M, Fournier-Del Castillo C, et al. Seizure and developmental outcomes after hemispherectomy in children and adolescents with intractable epilepsy. Childs Nerv Syst 2013;29:475-88. [PubMed]
- Barkovich AJ, Guerrini R, Kuzniecky RI, et al. A developmental and genetic classification for malformations of cortical development: update 2012. Brain 2012;135:1348-69. [PubMed]
- Barkovich AJ, Kuzniecky RI, Dobyns WB, et al. A classification scheme for malformations of cortical development. Neuropediatrics 1996;27:59-63. [PubMed]
- Palmini A, Lüders HO. Classification issues in malformations caused by abnormalities of cortical development. Neurosurg Clin N Am 2002;13:1-16. [PubMed]
- Wusthoff CJ, Kessler SK, Vossough A, et al. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics 2011;127:e1550-7.
- Kossoff EH, Vining EP, Pillas DJ, et al. Hemispherectomy for intractable unihemispheric epilepsy etiology vs outcome. Neurology 2003;61:887-90. [PubMed]
- Chen L, Feng P, Zhou D. Case reports of Rasmussen’s syndrome and literature review. J Neuropsychiatry Clin Neurosci 2012;24:367-71. [PubMed]
- Takahashi Y, Mori H, Mishina M, et al. Autoantibodies and cell-mediated autoimmunity to NMDA-type GluRepsilon2 in patients with Rasmussen’s encephalitis and chronic progressive epilepsia partialis continua. Epilepsia 2005;46 Suppl 5:152-8. [PubMed]
- Mantegazza R, Bernasconi P, Baggi F, et al. Antibodies against GluR3 peptides are not specific for Rasmussen’s encephalitis but are also present in epilepsy patients with severe, early onset disease and intractable seizures. J Neuroimmunol 2002;131:179-85. [PubMed]
- Bien CG, Urbach H, Deckert M, et al. Diagnosis and staging of Rasmussen’s encephalitis by serial MRI and histopathology. Neurology 2002;58:250-7. [PubMed]
- Lopinto-Khoury C, Sperling MR. Autoimmune status epilepticus. Curr Treat Options Neurol 2013;15:545-56. [PubMed]
- Villani F, Spreafico R, Farina L, et al. Positive response to immunomodulatory therapy in an adult patient with Rasmussen’s encephalitis. Neurology 2001;56:248-50. [PubMed]
- Leach JP, Chadwick DW, Miles JB, et al. Improvement in adult-onset Rasmussen’s encephalitis with long-term immunomodulatory therapy. Neurology 1999;52:738-42. [PubMed]
- Feichtinger M, Wiendl H, Körner E, et al. No effect of immunomodulatory therapy in focal epilepsy with positive glutamate receptor type 3-antibodies. Seizure 2006;15:350-4. [PubMed]
- Muto A, Oguni H, Takahashi Y, et al. Nationwide survey (incidence, clinical course, prognosis) of Rasmussen’s encephalitis. Brain Dev 2010;32:445-53. [PubMed]
- Lo W, Marchuk DA, Ball KL, et al. Updates and future horizons on the understanding, diagnosis, and treatment of Sturge-Weber syndrome brain involvement. Dev Med Child Neurol 2012;54:214-23. [PubMed]
- Bourgeois M, Crimmins DW, de Oliveira RS, et al. Surgical treatment of epilepsy in Sturge-Weber syndrome in children. J Neurosurg 2007;106:20-8. [PubMed]
- Tenney JR, Schapiro MB. Child neurology: hemiconvulsion-hemiplegia-epilepsy syndrome. Neurology 2012;79:e1-4. [PubMed]
- Caraballo R, Bartuluchi M, Cersósimo R, et al. Hemispherectomy in pediatric patients with epilepsy: a study of 45 cases with special emphasis on epileptic syndromes. Childs Nerv Syst 2011;27:2131-6. [PubMed]
- Jonas R, Nguyen S, Hu B, et al. Cerebral hemispherectomy: hospital course, seizure, developmental, language, and motor outcomes. Neurology 2004;62:1712-21. [PubMed]
- Moosa AN, Gupta A, Jehi L, et al. Longitudinal seizure outcome and prognostic predictors after hemispherectomy in 170 children. Neurology 2013;80:253-60. [PubMed]
- Ciliberto MA, Limbrick D, Powers A, et al. Palliative hemispherotomy in children with bilateral seizure onset. J Neurosurg Pediatr 2012;9:381-8. [PubMed]
- Lupashko S, Malik S, Donahue D, et al. Palliative functional hemispherectomy for treatment of refractory status epilepticus associated with Alpers’ disease. Childs Nerv Syst 2011;27:1321-3. [PubMed]
- Boshuisen K, van Schooneveld MM, Leijten FS, et al. Contralateral MRI abnormalities affect seizure and cognitive outcome after hemispherectomy. Neurology 2010;75:1623-30. [PubMed]
- Battaglia D, Chieffo D, Lettori D, et al. Cognitive assessment in epilepsy surgery of children. Childs Nerv Syst 2006;22:744-59. [PubMed]
- Lettori D, Battaglia D, Sacco A, et al. Early hemispherectomy in catastrophic epilepsy: a neuro-cognitive and epileptic long-term follow-up. Seizure 2008;17:49-63. [PubMed]
- Pulsifer MB, Brandt J, Salorio CF, et al. The cognitive outcome of hemispherectomy in 71 children. Epilepsia 2004;45:243-54. [PubMed]
- Dunkley C, Kung J, Scott RC, et al. Epilepsy surgery in children under 3 years. Epilepsy Res 2011;93:96-106. [PubMed]
- Thomas SG, Daniel RT, Chacko AG, et al. Cognitive changes following surgery in intractable hemispheric and sub-hemispheric pediatric epilepsy. Childs Nerv Syst 2010;26:1067-73. [PubMed]
- de Bode S, Firestine A, Mathern GW, et al. Residual motor control and cortical representations of function following hemispherectomy: effects of etiology. J Child Neurol 2005;20:64-75. [PubMed]
- Carson BS, Javedan SP, Freeman JM, et al. Hemispherectomy: a hemidecortication approach and review of 52 cases. J Neurosurg 1996;84:903-11. [PubMed]
- Davies KG, Maxwell RE, French LA. Hemispherectomy for intractable seizures: long-term results in 17 patients followed for up to 38 years. J Neurosurg 1993;78:733-40. [PubMed]
- Di Rocco C, Iannelli A. Hemimegalencephaly and intractable epilepsy: complications of hemispherectomy and their correlations with the surgical technique. A report on 15 cases. Pediatr Neurosurg 2000;33:198-207. [PubMed]
- González-Martínez JA, Gupta A, Kotagal P, et al. Hemispherectomy for catastrophic epilepsy in infants. Epilepsia 2005;46:1518-25. [PubMed]
- Lew SM, Matthews AE, Hartman AL, et al. Posthemispherectomy hydrocephalus: results of a comprehensive, multiinstitutional review. Epilepsia 2013;54:383-9. [PubMed]
- Kossoff EH, Vining EP, Pyzik PL, et al. The postoperative course and management of 106 hemidecortications. Pediatr Neurosurg 2002;37:298-303. [PubMed]
- Vadera S, Moosa AN, Jehi L, et al. Reoperative hemispherectomy for intractable epilepsy: a report of 36 patients. Neurosurgery 2012;71:388-92; discussion 392-3. [PubMed]